Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3 - PubMed (original) (raw)
Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-α/VEGF/Rho-GTPases: pivotal role of STAT-3
Srinivas Reddy Boreddy et al. PLoS One. 2011.
Abstract
Our previous studies have shown that benzyl isothiocyanate (BITC) suppresses pancreatic tumor growth by inhibiting STAT-3; however, the exact mechanism of tumor growth suppression was not clear. Here we evaluated the effects and mechanism of BITC on pancreatic tumor angiogenesis. Our results reveal that BITC significantly inhibits neovasularization on rat aorta and Chicken-Chorioallantoic membrane. Furthermore, BITC blocks the migration and invasion of BxPC-3 and PanC-1 pancreatic cancer cells in a dose dependant manner. Moreover, secretion of VEGF and MMP-2 in normoxic and hypoxic BxPC-3 and PanC-1 cells was significantly suppressed by BITC. Both VEGF and MMP-2 play a critical role in angiogenesis and metastasis. Our results reveal that BITC significantly suppresses the phosphorylation of VEGFR-2 (Tyr-1175), and expression of HIF-α. Rho-GTPases, which are regulated by VEGF play a crucial role in pancreatic cancer progression. BITC treatment reduced the expression of RhoC whereas up-regulated the expression of tumor suppressor RhoB. STAT-3 over-expression or IL-6 treatment significantly induced HIF-1α and VEGF expression; however, BITC substantially suppressed STAT-3 as well as STAT-3-induced HIF-1α and VEGF expression. Finally, in vivo tumor growth and matrigel-plug assay show reduced tumor growth and substantial reduction of hemoglobin content in the matrigel plugs and tumors of mice treated orally with 12 µmol BITC, indicating reduced tumor angiogenesis. Immunoblotting of BITC treated tumors show reduced expression of STAT-3 phosphorylation (Tyr-705), HIF-α, VEGFR-2, VEGF, MMP-2, CD31 and RhoC. Taken together, our results suggest that BITC suppresses pancreatic tumor growth by inhibiting tumor angiogenesis through STAT-3-dependant pathway.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
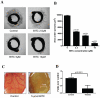
Figure 1. BITC inhibits angiogenesis ex vivo.
A. BITC inhibits VEGF-induced vessel sprouting ex vivo. Aortic rings (1 mm) were harvested from Sprague-Dawley rats, immerged in matrigel, and treated with VEGF (20 ng/mL) in the absence or presence of BITC (0, 2.5 and 5 and 10 µM) for 4 days and photographed under microscope (4X). Representative photographs are presented. B. Quantitative analysis of aortic ring assay. Aortic ring sprouting was quantified by Image J software and presented as mean ± SD of triplicates. C. Inhibition of CAM angiogenesis by BITC. Eggs were incubated at 37°C for 3 days. A Whatman filter disc containing the test compound (BITC 5 µmol) was placed on the CAM of eggs (n = 10) through pre-opened window and further incubated. On day 9–12 of incubation, photographs were made after removing the filter discs. A representative photograph is presented. D. Blood vessels density was quantified by Image J software and represented as a bar diagram.
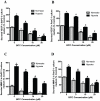
Figure 2. BITC inhibits secretion of proangiogenic factors from BxPC-3 and PanC-1 cells under normoxic and hypoxic conditions.
Serum-starved BxPC-3 or PanC-1 cells were treated with various concentrations of BITC in a 96-well plate and incubated for 24 h. For hypoxia treatment, cells were exposed to 1% pO2 for 24 h. Culture supernatants were collected and assayed for MMP-2 or VEGF by ELISA kit -. A–B. BITC suppresses secretion of VEGF from BxPC-3 and PanC-1 cells. C–D. BITC blocks secretion of MMP-2. Values are mean ± SD of triplicates. *p<0.01 statistically significant when compared with normoxic controls. #p<0.01, statistically significant when compared with hypoxic controls.
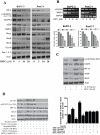
Figure 3. BITC suppresses proangiogenic proteins.
A. BITC down-regulates expression of HIF-α, VEGFR-2, Rho-GTPase signaling molecules. BxPC-3 or PanC-1 cells were treated with various concentrations of BITC for 24 h and for HIF-α, cells were exposed to 1% O2 for 12 h. Whole cell lysates were prepared with urea-CHAPS buffer. Cell lysates were analyzed by SDS-PAGE followed by western blot. B. BITC down-regulates mRNA levels of VEGFR2 and MMP-2 in BxPC-3 and PanC-1 cells. Cells were treated with different concentrations of BITC and total RNA was isolated with Trizol. Total RNA was analyzed for the expression levels of VEGFR-2 and MMP-2 by RT-PCR. GAPDH was used as internal control. Quantitative analysis of mRNA expression levels were performed by Image J software and presented as bar diagram (lower panel) C. BITC inhibits HIF-1α and VEGF expression by inhibiting phosphorylation of STAT-3. 0.3×106 cells were plated in 6-well plate and treated with 20 ng/mL IL-6 and 10 µM BITC for 24 h. Cells were analyzed for STAT-3 (Tyr 705), STAT-3, HIF-1α, VEGF, and RhoC expression by western blot. D. STAT-3 is required for BITC mediated inhibition of HIF-1α and VEGF expression. BxPC-3 cells were transfected with 2 µg of STAT-3α over-expressing plasmid and, in another experiment, STAT-3 was either transiently silenced or permanently knocked out by shRNA. Transfected cells were treated with or without 10 µM BITC for 24 h after 48 h of transfection. Cells were lysed and analyzed by western blot. Right panel showing secreted VEGF level was evaluated in STAT-3 over-expressing or silenced BxPC-3 cells by ELISA as described above. * Values of p<0.01 statistically significant when compared with normoxic controls. **p<0.01 statistically significant when compared with hypoxic controls. #p<0.01statistically significant when compared with STAT-3 over-expressing cells.
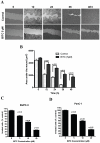
Figure 4. BITC inhibits migration and invasion of pancreatic cancer cells.
A. BITC inhibits migration of BxPC-3 cells. BxPC-3 cells were plated, scratched with pipette tip, and incubated in the absence or presence of 5 µM BITC. Photomicrographs were made at regular intervals using inverted microscope. B. Quantitative representation of migration assay. Wound area in BITC-treated and control cells were quantified by Image J software and presented as mean ± SD of triplicates. p<0.01, statistically significant when compared to corresponding time points in controls cells. C–D. BITC inhibits the invasion of BxPC-3 and PanC-1 cells. Invasion assay was performed using Boyden's chamber. Results are presented as mean ± SD of triplicates. p<0.05, statistically significant when compared controls.
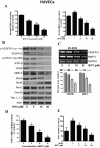
Figure 5. BITC inhibits angiogenesis in HUVECs.
A. BITC inhibits the secretion of proangiogenic factors from HUVECs. Cells were plated, stimulated with VEGF, and treated with BITC for 24 h. Media was collected and assayed for MMP-2 and VEGF by ELISA kit. *p<0.01 statistically significant when compared with controls. #p<0.01 statistically significant when compared with VEGF-stimulated controls. B. Regulation of VEGF mediated signaling by BITC. HUVECs were treated with various concentrations of BITC and whole cell lysates were analyzed by western blot. C. BITC down-regulates VEGFR2 and MMP-2 mRNA in HUVECs. Total RNA from BITC-treated HUVECs was isolated with Trizol and analyzed for the expression levels of VEGFR-2 and MMP-2 by RT-PCR. GAPDH was used as internal control. mRNA expression levels were quantified by Image J software and presented as bar diagram (lower panel). D. BITC inhibits STAT-3 DNA binding activity in HUVECs. HUVECs were treated with BITC and nuclear fraction was collected. Around 5 µg of nuclear protein subjected to STAT-3 DNA binding activity by Universal EZ-TFA transcription factor assay colorimetric kit according to the manufacturer's protocol. #p<0.01 statistically significant when compared with controls. E. BITC inhibits invasion of HUVECs. Invasion assay was performed using Boyden's chamber. *p<0.01 statistically significant when compared with controls. #p<0.01 statistically significant when compared with VEGF-stimulated controls
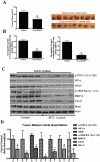
Figure 6. BITC inhibits in vivo tumor growth by inhibiting proangiogenic proteins.
A. BITC suppresses tumor growth in vivo. BxPC-3 xenografts bearing mice (n = 10) were orally fed with 12 µmol BITC daily for 40 days. Right side panel shows photographs of isolated tumors from control and BITC-treated mice. B. BITC inhibits tumor angiogenesis. BxPC-3 xenografts or matrigel plug-bearing mice were fed with 12 µmol BITC daily for 40 or 7 days, respectively. Tumors and plugs were collected and 50 mg of tumor or plugs were analyzed for hemoglobin content by Drabkin's reagent. C. BITC down-regulates pro-angiogenic proteins in tumor xenografts. Cell lysates were prepared from isolated tumor xenografts, subjected to western blot and analyzed for VEGFR-2, MMP-2, HIF-α, and Rho-GTPases D. Quantitative analysis of tumor western blots. *p<0.01 statistically significant when compared with controls.
Similar articles
- The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate.
Sahu RP, Srivastava SK. Sahu RP, et al. J Natl Cancer Inst. 2009 Feb 4;101(3):176-93. doi: 10.1093/jnci/djn470. Epub 2009 Jan 27. J Natl Cancer Inst. 2009. PMID: 19176463 Free PMC article. - Magnolol suppresses hypoxia-induced angiogenesis via inhibition of HIF-1α/VEGF signaling pathway in human bladder cancer cells.
Chen MC, Lee CF, Huang WH, Chou TC. Chen MC, et al. Biochem Pharmacol. 2013 May 1;85(9):1278-87. doi: 10.1016/j.bcp.2013.02.009. Epub 2013 Feb 14. Biochem Pharmacol. 2013. PMID: 23416116 - AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1α/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo.
Zhang PC, Liu X, Li MM, Ma YY, Sun HT, Tian XY, Wang Y, Liu M, Fu LS, Wang YF, Chen HY, Liu Z. Zhang PC, et al. Biochem Pharmacol. 2020 Feb;172:113771. doi: 10.1016/j.bcp.2019.113771. Epub 2019 Dec 18. Biochem Pharmacol. 2020. PMID: 31863779 - A Molecular Perspective on HIF-1α and Angiogenic Stimulator Networks and Their Role in Solid Tumors: An Update.
Magar AG, Morya VK, Kwak MK, Oh JU, Noh KC. Magar AG, et al. Int J Mol Sci. 2024 Mar 14;25(6):3313. doi: 10.3390/ijms25063313. Int J Mol Sci. 2024. PMID: 38542288 Free PMC article. Review. - Mast cells and angiogenesis in pancreatic ductal adenocarcinoma.
Longo V, Tamma R, Brunetti O, Pisconti S, Argentiero A, Silvestris N, Ribatti D. Longo V, et al. Clin Exp Med. 2018 Aug;18(3):319-323. doi: 10.1007/s10238-018-0493-6. Epub 2018 Feb 28. Clin Exp Med. 2018. PMID: 29492715 Review.
Cited by
- Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells.
Mimeault M, Batra SK. Mimeault M, et al. J Cell Mol Med. 2013 Jan;17(1):30-54. doi: 10.1111/jcmm.12004. Epub 2013 Jan 10. J Cell Mol Med. 2013. PMID: 23301832 Free PMC article. - Gene-gene interactions and gene polymorphisms of VEGFA and EG-VEGF gene systems in recurrent pregnancy loss.
Su MT, Lin SH, Chen YC, Kuo PL. Su MT, et al. J Assist Reprod Genet. 2014 Jun;31(6):699-705. doi: 10.1007/s10815-014-0223-2. Epub 2014 Mar 27. J Assist Reprod Genet. 2014. PMID: 24671265 Free PMC article. - Chronic inflammation and cytokines in the tumor microenvironment.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Landskron G, et al. J Immunol Res. 2014;2014:149185. doi: 10.1155/2014/149185. Epub 2014 May 13. J Immunol Res. 2014. PMID: 24901008 Free PMC article. Review. - Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis.
Gupta N, Srivastava SK. Gupta N, et al. Pharmaceuticals (Basel). 2021 May 28;14(6):521. doi: 10.3390/ph14060521. Pharmaceuticals (Basel). 2021. PMID: 34071408 Free PMC article. - Molecular targets of isothiocyanates in cancer: recent advances.
Gupta P, Kim B, Kim SH, Srivastava SK. Gupta P, et al. Mol Nutr Food Res. 2014 Aug;58(8):1685-707. doi: 10.1002/mnfr.201300684. Epub 2014 Feb 10. Mol Nutr Food Res. 2014. PMID: 24510468 Free PMC article. Review.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin 2010 - PubMed
- Philip PA. Targeting angiogenesis in pancreatic cancer. Lancet. 2008;371:2062–2064. - PubMed
- Wei D, Wang L, He Y, Xiong HQ, Abbruzzese JL, et al. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity. Cancer Res. 2004;64:2030–2038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous