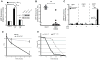Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway - PubMed (original) (raw)
. 2011 Oct 28;35(4):536-49.
doi: 10.1016/j.immuni.2011.08.015. Epub 2011 Oct 20.
Lorin Magoc, Stephanie Dejardin, Michael Cappillino, Nicholas Paquette, Charlotte Hinault, Guillaume M Charriere, W K Eddie Ip, Shannon Fracchia, Elizabeth Hennessy, Deniz Erturk-Hasdemir, Jean-Marc Reichhart, Neal Silverman, Adam Lacy-Hulbert, Lynda M Stuart
Affiliations
- PMID: 22018470
- PMCID: PMC3258503
- DOI: 10.1016/j.immuni.2011.08.015
Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway
Laurent Boyer et al. Immunity. 2011.
Abstract
Although infections with virulent pathogens often induce a strong inflammatory reaction, what drives the increased immune response to pathogens compared to nonpathogenic microbes is poorly understood. One possibility is that the immune system senses the level of threat from a microorganism and augments the response accordingly. Here, focusing on cytotoxic necrotizing factor 1 (CNF1), an Escherichia coli-derived effector molecule, we showed the host indirectly sensed the pathogen by monitoring for the effector that modified RhoGTPases. CNF1 modified Rac2, which then interacted with the innate immune adaptors IMD and Rip1-Rip2 in flies and mammalian cells, respectively, to drive an immune response. This response was protective and increased the ability of the host to restrict pathogen growth, thus defining a mechanism of effector-triggered immunity that contributes to how metazoans defend against microbes with pathogenic potential.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
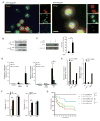
Figure 1. _E. coli_-derived CNF1 induces effector-triggered immunity in the absence of other bacterial components and contributes to host resistance in vivo
(A) In vitro induction of Drosomycin (black bars) and Diptericin (white bars) after transfection of catalytically active C-terminus of CNF1 (CNF1CT) and the inactive point mutant (CNF1CS) in Drosophila S2 cells. Insert shows equivalent expression of WT and mutant toxin C-termini. (B) In vivo induction of _Drosomycin_-GFP in reporter flies injected with purified CNF1 (10−5M) or inactive point mutant C866S (CNF1C866S) control toxin. Data expressed as integrated total GFP fluorescence per fly. (C) In vivo induction of AMPs of by heat shock (HS) in flies expressing UAS-GFP or the catalytically active C-terminus of CNF1 (CNF1CT) and inactive point mutant (CNF1CS) under the control of an HSP70-Gal4, tubulin-Gal80ts inducible driver. Data representative of mean+/− s.d. of individual flies in one experiment and representative of experiments using 3 or more independent CNF1CT or CNF1CS transgenic insertion lines. (D) In vivo bacterial loads in OR flies infected with E. coli J96 (black) or mutants lacking CNF1 (grey) at the indicated times. Mean+/− range of c.f.u. per fly calculated at two different dilutions. Data representative of >3 similar experiments. (E) In vivo survival of CNF1CT or control CNF1CS flies infected with Pseudomonas PA14. *p<0.05, **p<0.01.
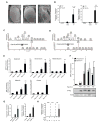
Figure 2. CNF1 modifies the small RhoGTPase, Rac2, which induces a defense response
(A) Absence of the ommatidia and a severe rough eye phenotype due to ectopic expression of the toxin in GMR-Gal4/UAS-CNF1CT flies but not control or GMR-Gal4/UAS-CNF1CS flies. (B) In vivo intoxication of WT (black bars) or Rac2Δ (white bars) flies with purified CNF1 toxin and AMPs measured at 6 hours. Drosomycin and Diptericin expression profiles were quantified by QRT-PCR in pools of 3 or more flies (mean+/− s.e.m. of 6 pools from 3 independent experiments). (C & D) Mass spectrometry chromatograms of (C) Native GST-Rac2 and (D) CNF1 modified GST-Rac2. The upper panel represents the total ion chromatogram of GST-Rac2 before (C) or after (D) CNF1 modification. Lower panels show the extracted ion chromatogram for the peptide that includes the position 61 of Rac2 targeted by CNF1 before (C) or after (D) CNF1 modification. The predicted protein sequences are shown. (E) In vitro expression of Diptericin, Drosomycin, Metchnikowin, AttacinA, CecropinA in S2 cells 16h after transfection with Flag-Rac2, Flag-Rac2L61, Flag-Rac2N17 or Flag plasmid (control). AMPs were monitored using QRT-PCR, normalized to RP49 and expressed relative to the empty flag construct (mean+/− s.d.). (F) The upper panel represents the in vitro expression of Diptericin and Drosomycin, in S2 cells 16h after transfection with Flag-Rac2N17, Flag-Rac2L61, Flag-Rac2E61, Flag-Rac2V12. The lower panel is an immunoblot analysis showing levels of expression of the Flag-Rac2 mutants (G) In vitro AMP expression induced by transfection of S2 cells with Rac2L61, Toll10b and PGRP-LC (mean+/− s.d.). (H) In vivo induction of AMPs of by heat shock (HS) in flies expressing UAS-GFP or active Rac2 (Rac2L61) and inactive mutant (Rac2N17) under the control of an HSP70-Gal4, tubulin-Gal80ts inducible driver. Data represent the mean+/− s.e.m. of ‘n’ individual flies pooled from 3 or more independent experiments and representative of results using 3 or more independent Rac2L61 or Rac2N17 transgenic insertion lines. *p<0.05, **p<0.01
Figure 3. Rac2 induces an immune response independently of cytoskeletal changes
(A) Cell morphology of S2 cells expressing Flag-Rac2L61 and switch mutant, Flag-Rac2L61,C40, but not Flag-Rac2L61,A37. Large-scale image is actin (phalloidin-FITC). Smaller images show red, anti-Flag; blue, DAPI and the merge with green, actin (phalloidin-FITC). Scale bar 10μm. (B) In vitro AMP expression in S2 cells expressing Flag-Rac2L61 and switch mutant, Flag-Rac2L61,A37, but not Flag-Rac2L61,C40(mean+/− s.d). Insert demonstrating equivalent expression of all constructs. (C) In vitro AMP expression induced in S2 cells by expressing Flag-Rac2L61 and the ΔCAAX mutant (mean+/− s.d). *p<0.05, **p<0.01.
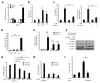
Figure 4. Modified Rac2 induces activation of NF-kB-like innate immune transcription factors through an IMD-dependent mechanism
(A) Immunoblot of Cactus in stable S2 cell lines expressing Rac2L61. Streptavidin–HRP staining indicates levels of BioeaseRac2L61 induced by increasing concentration of CuSO4 as indicated. Actin staining is shown as a loading control. (B) In vitro localization of Dorsal was assayed by blind counting of cells co-transfected with GFP-Dorsal and the Flag-tagged Rac2 mutants, Rac2L61 (active) or Rac2N17 (inactive). (C) Immunoblot showing levels of the transcription factor Relish in S2 cells 16h after transient transfection with Flag-Rac2, Flag-Rac2L61, Flag-Rac2N17, Flag empty plasmid (Ctrl), PGRP-LC or Toll10b plasmids. Flag-tag immunostaining indicates expression of Rac2 proteins. Relish activation was determined by detection of the 68kD phosphorylated, cleaved fragment. Actin staining is shown as a loading control. (D) In vitro localization of Relish localization was assayed by blind counting of cells co-transfected with YFP-Relish and the mRFP tagged Rac2 mutants, Rac2L61 and Rac2N17. (E) Drosomycin (black bars) and Diptericin (grey bars) expression profile were monitored in BioeaseRac2L61 stable S2 cells treated with the indicated RNAi. BioeaseRac2L61 stable cells treated with a GFP RNAi without CuSO4 induction and induced with 500μM CuSO4 are used as controls in each experiment. AMPs expression was determined QRT-PCR and normalized to the house-keeping gene, RP49. Results expressed as % of the induced cells treated with GFP RNAi, (mean+/− s.d.) of 3 independent screens. *, p<0.05, student T test.
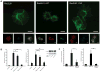
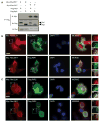
Figure 5. Modified Rac2 interacts with the innate immune adaptor IMD to induce AMPs
(A) Immunofluorescence of S2 cells transfected with mRFP-Rac2L61 or Rac2N17 (red) and colocalization with endogenous IMD (green) determined by antibody staining 16h after transfection. Blue, nuclei (DAPI). (B) IMD interaction with Rac2 were determined by Biotin-Streptavidin based pull-down of biotinylated Rac2 from BioeaseRac2L61 and BioeaseRac2N17 stable S2 cells. BioeaseLacZ acted as a negative control. 2% of total cell lysate before streptavidin purification was loaded on the gel (Input) or proteins eluted after streptavidin pull-down purification (PD). Endogenous IMD was detected by immunostaining. Transfection and pull-down efficiency for the two BioeaseRac2 mutants is shown by Streptavidin–HRP staining of the input and PD specimens respectively. (C) Binding of native GST-Rac2 or GST-Rac2 modified by CNF1 to IMD in S2 cell lysates was determined by immunoblotting. Densitometry of IMD normalized for GST-Rac2 pull-down efficiency in three independent experiments (mean+/− s.d.). (D) In vivo AMPs response to CNF1 was measured in pools of WT (black bars), imd1 (dark blue bars) and _imd_shadok (green bars) flies intoxicated with purified toxin and measured at 6 hours. Drosomycin and Diptericin expression profiles were quantified by QRT-PCR in pools containing 3 or more flies (mean+/− s.e.m. of 6 pools from 3 independent experiments). *p<0.05, **p<0.01. (E) In vivo AMPs response to CNF1 was measured in pools of WT, _imd_shadok and Myd88 flies intoxicated with purified toxin and measured at 6 hours. Drosomycin and Diptericin expression profiles were quantified by QRT-PCR in pools containing 3 or more flies (data representative of 2 similar experiments). (F) In vivo bacterial loads in WT, Rac2Δ (left) or imd1 (right) flies infected with E. coli J96 (white) or mutants lacking CNF1 (black). Mean+/− range of c.f.u. per fly. Data representative of 2 or more similar experiments. (G) Survival or WT, imd1 or PGRP-LC+LE double mutant flies (15–25 per group) infected with E. coli K12-CNF1 or E. coli K12 CNF1-C866S. *p<0.05 using a Gehan-Breslow-Wilcoxon Chi squared Test
Figure 6. Modified human Rac2 interacts with the IMD-related molecules, Rip1 and Rip2
(A) Co-immunoprecipitation of myc-hRac2L61 or hRac2N17 with Flag-Rip1 or Rip2 expressed in HEK293T cells. B & C) Immunofluorescence showing cells co-transfected with a plasmid expressing Myc-tagged hRac2 mutants hRac2L61 or hRac2N17 and the Flag-Rip1 (B) or Flag-Rip2 (C). Red, anti-Myc and green, anti-Flag antibodies. Blue, Nuclei (DAPI). Scale bar, 10μm. High magnification images of indicated fields are shown in inserts.
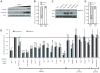
Figure 7. CNF1 induces an immune response after modification of human Rac2 that induces signaling via IMD-related molecules, Rip1 and Rip2
(A) NF-kB activation in control HEK 293T cells (white bars), cells exposed to purified heat inactivated CNF1 (grey bars) and CNF1 (black bars) (mean+/− s.d.). (B) IL-8 induction by CNF1-C866S (CS) or CNF1(CT) intoxication or by the addition of 100ng/ml LPS. (C) CNF1 induction of IL-8, after 24h of intoxication, in the presence or absence hRac2N17 that functions as a dominant negative inhibitor of Rac. (D) NF-kB induction and (E) cytokine expression triggered by hRac2 mutants, hRac2L61 or hRac2N17, expression in HEK 293T cells (mean+/− s.e.m., n=3). F-H Rip1 or Rip2 or a Non Targeting (NT) siRNA were transfected in HEK 293T cells 72h as indicated. (F) Endogenous Rip1 and Rip2 immunoblot demonstrates siRNA efficiency and specificity of the siRNA. Anti-Myc staining demonstrates levels of transfection of Myc-hRac2 mutants. Actin staining was used as a loading control. (G) Quantification of hRac2L61 induced NF-kB activation in Rip1 and Rip2 knocked down cells. NF-kB Firefly-luciferase in total cellular lysates was measured and transfection efficiency of the reporter normalized using Renilla activity. Results expressed as % of hRac2L61 expressing cells, mock transfected with siRNA. (H) IL-8 gene expression monitored by QRT-PCR after siRNAi transfection of HEK 293T cells expressing Myc-hRac2 mutants as described above. Actin was used as housekeeping gene and internal control and data expressed normalized to untreated cells. Data is from cells grown in triplicate wells and representative of three independent experiments. (I) CNF1 induction of IL-8, after 24h of intoxication, in the presence or absence RIP1 and RIP2 knock-down. All data are shown as the mean+/− s.d. of biological triplicates and representative of three independent experiments unless otherwise stated. *p<0.05, **p<0.01.
Similar articles
- RIP kinases: key decision makers in cell death and innate immunity.
Humphries F, Yang S, Wang B, Moynagh PN. Humphries F, et al. Cell Death Differ. 2015 Feb;22(2):225-36. doi: 10.1038/cdd.2014.126. Epub 2014 Aug 22. Cell Death Differ. 2015. PMID: 25146926 Free PMC article. Review. - Rac2 regulates neutrophil chemotaxis, superoxide production, and myeloid colony formation through multiple distinct effector pathways.
Carstanjen D, Yamauchi A, Koornneef A, Zang H, Filippi MD, Harris C, Towe J, Atkinson S, Zheng Y, Dinauer MC, Williams DA. Carstanjen D, et al. J Immunol. 2005 Apr 15;174(8):4613-20. doi: 10.4049/jimmunol.174.8.4613. J Immunol. 2005. PMID: 15814684 - TRIP6 is a RIP2-associated common signaling component of multiple NF-kappaB activation pathways.
Li L, Bin LH, Li F, Liu Y, Chen D, Zhai Z, Shu HB. Li L, et al. J Cell Sci. 2005 Feb 1;118(Pt 3):555-63. doi: 10.1242/jcs.01641. Epub 2005 Jan 18. J Cell Sci. 2005. PMID: 15657077 - Rac2 is a major actor of Drosophila resistance to Pseudomonas aeruginosa acting in phagocytic cells.
Avet-Rochex A, Perrin J, Bergeret E, Fauvarque MO. Avet-Rochex A, et al. Genes Cells. 2007 Oct;12(10):1193-204. doi: 10.1111/j.1365-2443.2007.01121.x. Genes Cells. 2007. PMID: 17903178 - Mechanisms and pathways of innate immune activation and regulation in health and cancer.
Cui J, Chen Y, Wang HY, Wang RF. Cui J, et al. Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
Cited by
- RAC2 gain-of-function variants causing inborn error of immunity drive NLRP3 inflammasome activation.
Doye A, Chaintreuil P, Lagresle-Peyrou C, Batistic L, Marion V, Munro P, Loubatier C, Chirara R, Sorel N, Bessot B, Bronnec P, Contenti J, Courjon J, Giordanengo V, Jacquel A, Barbry P, Couralet M, Aladjidi N, Fischer A, Cavazzana M, Mallebranche C, Visvikis O, Kracker S, Moshous D, Verhoeyen E, Boyer L. Doye A, et al. J Exp Med. 2024 Oct 7;221(10):e20231562. doi: 10.1084/jem.20231562. Epub 2024 Aug 30. J Exp Med. 2024. PMID: 39212656 - Effects of the Escherichia coli Bacterial Toxin Cytotoxic Necrotizing Factor 1 on Different Human and Animal Cells: A Systematic Review.
Carlini F, Maroccia Z, Fiorentini C, Travaglione S, Fabbri A. Carlini F, et al. Int J Mol Sci. 2021 Nov 22;22(22):12610. doi: 10.3390/ijms222212610. Int J Mol Sci. 2021. PMID: 34830494 Free PMC article. Review. - Inflammation and colorectal cancer, when microbiota-host mutualism breaks.
Candela M, Turroni S, Biagi E, Carbonero F, Rampelli S, Fiorentini C, Brigidi P. Candela M, et al. World J Gastroenterol. 2014 Jan 28;20(4):908-22. doi: 10.3748/wjg.v20.i4.908. World J Gastroenterol. 2014. PMID: 24574765 Free PMC article. Review. - Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells.
Orzalli MH, Prochera A, Payne L, Smith A, Garlick JA, Kagan JC. Orzalli MH, et al. Immunity. 2021 Jul 13;54(7):1447-1462.e5. doi: 10.1016/j.immuni.2021.04.012. Epub 2021 May 11. Immunity. 2021. PMID: 33979579 Free PMC article. - Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns.
Benedetti M, Pontiggia D, Raggi S, Cheng Z, Scaloni F, Ferrari S, Ausubel FM, Cervone F, De Lorenzo G. Benedetti M, et al. Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5533-8. doi: 10.1073/pnas.1504154112. Epub 2015 Apr 13. Proc Natl Acad Sci U S A. 2015. PMID: 25870275 Free PMC article.
References
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. - PubMed
- Aktories K, Schmidt G, Just I. Rho GTPases as targets of bacterial protein toxins. Biol Chem. 2000;381:421–426. - PubMed
- Avet-Rochex A, Perrin J, Bergeret E, Fauvarque MO. Rac2 is a major actor of Drosophila resistance to Pseudomonas aeruginosa acting in phagocytic cells. Genes Cells. 2007;12:1193–1204. - PubMed
- Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P01 AI044220/AI/NIAID NIH HHS/United States
- R01A160025/PHS HHS/United States
- R01 AI079198/AI/NIAID NIH HHS/United States
- P01A104420/PHS HHS/United States
- R01 AI060025/AI/NIAID NIH HHS/United States
- P01 AI044220-09/AI/NIAID NIH HHS/United States
- R01 AI079198-04/AI/NIAID NIH HHS/United States
- R01 AI060025-05/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous