Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice - PubMed (original) (raw)
Comparative Study
Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice
Friederike Klempin et al. PLoS One. 2011.
Abstract
The piriform cortex receives input from the olfactory bulb and (via the entorhinal cortex) sends efferents to the hippocampus, thereby connecting the two canonical neurogenic regions of the adult rodent brain. Doublecortin (DCX) is a cytoskeleton-associated protein that is expressed transiently in the course of adult neurogenesis. Interestingly, the adult piriform cortex, which is usually considered non-neurogenic (even though some reports exist that state otherwise), also contains an abundant population of DCX-positive cells. We asked how similar these cells would be to DCX-positive cells in the course of adult hippocampal neurogenesis. Using BAC-generated transgenic mice that express GFP under the DCX promoter, we studied DCX-expression and electrophysiological properties of DCX-positive cells in the mouse piriform cortex in comparison with the dentate gyrus. While one class of cells in the piriform cortex indeed showed features similar to newly generated immature granule neurons, the majority of DCX cells in the piriform cortex was mature and revealed large Na+ currents and multiple action potentials. Furthermore, when proliferative activity was assessed, we found that all DCX-expressing cells in the piriform cortex were strictly postmitotic, suggesting that no DCX-positive "neuroblasts" exist here as they do in the dentate gyrus. We conclude that DCX in the piriform cortex marks a unique population of postmitotic neurons with a subpopulation that retains immature characteristics associated with synaptic plasticity. DCX is thus, per se, no marker of neurogenesis but might be associated more broadly with plasticity.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
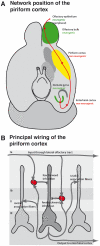
Figure 1. Localization and wiring of the piriform cortex.
(A) Position of the piriform cortex in the circuitry of the olfactory system. The schematic drawing is based on information from . (B) Principal network of the piriform cortex.
Figure 2. DCX-GFP expression and its distribution in the adult brain.
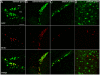
(A–B) GFP (in green) is expressed abundantly in progenitor cells of the adult dentate gyrus (A) and subventricular zone (B) that also incorporated BrdU (red). (C–D) GFP is not only confined to neurogenic regions but also observed in the stratum oriens of the CA1 field of the hippocampal formation (C) where also proliferating cells could be detected (BrdU in red), and the adult piriform cortex (D). Notably, here no proliferation of GFP+ cells was observed at either time.
Figure 3. Transgenic DCX-GFP expression in comparison with DCX-antigen labeling in the adult mouse DG.
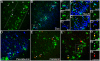
(A–B) DAB immunoreactivity reveals strong staining in nucleus, soma and proximal processes of GFP+ cells (A) in the granule cell layer (GCL) and weak expression in dendritic trees branching into the molecular layer (ML) relative to DCX-protein (B). A few cells had migrated into the inner GCL (arrow in A); Scale bar 40 µm. (C) Confocal images of GFP expression reveals strong staining of progenitor cells in the DG (arrows), and some weak labeling in hilar interneurons (asterisk). (C1) GFP and DCX-antigen labeling matches to approximately 90%, but GFP is down regulated in more mature neurons although DCX-protein is still present (arrowhead). Scale bar 100 µm.
Figure 4. Phenotypes of DCX-GFP-expressing cells in the adult DG.
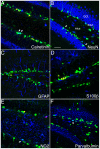
(A–B) In the course of adult neurogenesis some of GFP+ cells co-express the early transient postmitotic marker Calretinin (CR, in A) and the lasting postmitotic neuronal marker NeuN (B), indicating that DCX is present in immature neurons. DCX-GFP-positive cells with rounded or flattened nuclear morphology, negative for the other markers represent the precursor cells at the type-2b and type-3 stage (compare [14]). DCX-GFP is absent from the radial glia-like neural stem cells (type-1 cells) and astrocytes as detected with GFAP (C) and S100β (D). S100β-positive cells represent postmitotic astrocytes, some of which are produced in the course of adult neurogenesis. (E) A few GFP+ cells express NG2 near to the subgranular zone (SGZ). (F) Some of the fainter GFP+ cells in the hilus are colabeled with the interneuron marker Parvalbumin. GCL, granule cell layer; BrdU in red; Scale bar, 120 µm.
Figure 5. Phenotypic marker expression and distribution of DCX-GFP cells in the adult piriform cortex (Bregma –0.82/2.98).
(A) Bright and abundant GFP expression is observed in neurogliaform cells in layer II in close proximity to layer I, whereas semilunar-pyramidal neurons (arrows in layer II) and deep/large pyramidal neurons (arrowheads in layer III) show partly faint GFP signaling. (B) DCX-protein (in blue) is mainly expressed by neurogliaform cells that often form clusters (arrowheads) around semilunar pyramidal neurons (arrow) with only weak GFP but strong DCX-protein expression (B1–B3); the broken arrow displays a semilunar-pyramidal neuron with stronger GFP signaling, while no DCX overlap was found in morphology-wise interneuron populations (asterisk). (C, C′) NeuN (in blue) is expressed by all deep/large pyramidal neurons (DP) with the typical apical dendrites (small arrows), and by approximately 60% of semilunar-pyramidal neurons (SP; C1–C3). Only a few neurogliaform cells (NG), and some horizontal (H) interneurons are NeuN+. (D–E) Some GFP+ cells in layer II and III express the interneuron marker Parvalbumin (in blue, arrow), and Calretinin (in red, arrows), in addition to a third of neurogliaform cells that are also CR+ (E′, E1-3), asterisk marks CR+ but GFP- cells. Scale bar 150 µm.
Figure 6. Physiological properties of DCX-GFP-expressing cells in the adult piriform cortex in comparison to the DG.
GFP+ cells (green) from adult mouse brain slices were patch-clamped with a glass pipette and filled with 10 µg/ml Alexa Fluor 594 (red). (A) Images of a neurogliaform cell, a semilunar-pyramidal neuron, and a large pyramidal neuron in the piriform cortex; bright and weak cells in the dentate gyrus. The patched cell is enlarged shown in a small box above. Cortical layers are marked as I, II, and III. (B) Large Na+ currents were detected in deep pyramidal neurons, whereas neurogliaform cells and dentate newly born neurons had small Na+ currents. (C) Single and multiple action potentials were elicited by 200 ms current injection of 30 and 80 pA in the piriform cortex and dentate gyrus, respectively. (D) Spontaneous current were detected in a proportion of semilunar-pyramidal neurons and large pyramidal neurons of the piriform cortex but not in newly generated cells of the dentate gyrus; NG, neurogliaform cells, SP, semilunar-pyramidal neurons, DP, large/deep pyramidal neurons.
Similar articles
- Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus.
Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, Page CE, Sandoval K, Knox A, Connolly A, Huang EJ, Garcia-Verdugo JM, Oldham MC, Yang Z, Alvarez-Buylla A. Sorrells SF, et al. J Neurosci. 2021 Mar 24;41(12):2554-2565. doi: 10.1523/JNEUROSCI.0676-20.2020. J Neurosci. 2021. PMID: 33762407 Free PMC article. Review. - Transient expression of doublecortin during adult neurogenesis.
Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Brown JP, et al. J Comp Neurol. 2003 Dec 1;467(1):1-10. doi: 10.1002/cne.10874. J Comp Neurol. 2003. PMID: 14574675 - Evidence that Doublecortin is dispensable for the development of adult born neurons in mice.
Merz K, Lie DC. Merz K, et al. PLoS One. 2013 May 7;8(5):e62693. doi: 10.1371/journal.pone.0062693. Print 2013. PLoS One. 2013. PMID: 23667508 Free PMC article. - Cellular Plasticity in the Adult Murine Piriform Cortex: Continuous Maturation of Dormant Precursors Into Excitatory Neurons.
Rotheneichner P, Belles M, Benedetti B, König R, Dannehl D, Kreutzer C, Zaunmair P, Engelhardt M, Aigner L, Nacher J, Couillard-Despres S. Rotheneichner P, et al. Cereb Cortex. 2018 Jul 1;28(7):2610-2621. doi: 10.1093/cercor/bhy087. Cereb Cortex. 2018. PMID: 29688272 Free PMC article. - Postnatal and Adult Neurogenesis in Mammals, Including Marsupials.
Bartkowska K, Tepper B, Turlejski K, Djavadian R. Bartkowska K, et al. Cells. 2022 Sep 1;11(17):2735. doi: 10.3390/cells11172735. Cells. 2022. PMID: 36078144 Free PMC article. Review.
Cited by
- A hypothesis for the evolution of the upper layers of the neocortex through co-option of the olfactory cortex developmental program.
Luzzati F. Luzzati F. Front Neurosci. 2015 May 12;9:162. doi: 10.3389/fnins.2015.00162. eCollection 2015. Front Neurosci. 2015. PMID: 26029038 Free PMC article. - Positive Controls in Adults and Children Support That Very Few, If Any, New Neurons Are Born in the Adult Human Hippocampus.
Sorrells SF, Paredes MF, Zhang Z, Kang G, Pastor-Alonso O, Biagiotti S, Page CE, Sandoval K, Knox A, Connolly A, Huang EJ, Garcia-Verdugo JM, Oldham MC, Yang Z, Alvarez-Buylla A. Sorrells SF, et al. J Neurosci. 2021 Mar 24;41(12):2554-2565. doi: 10.1523/JNEUROSCI.0676-20.2020. J Neurosci. 2021. PMID: 33762407 Free PMC article. Review. - Expression of progenitor cell/immature neuron markers does not present definitive evidence for adult neurogenesis.
Hagihara H, Murano T, Ohira K, Miwa M, Nakamura K, Miyakawa T. Hagihara H, et al. Mol Brain. 2019 Dec 10;12(1):108. doi: 10.1186/s13041-019-0522-8. Mol Brain. 2019. PMID: 31823803 Free PMC article. Review. - Age-related changes in layer II immature neurons of the murine piriform cortex.
Ghibaudi M, Marchetti N, Vergnano E, La Rosa C, Benedetti B, Couillard-Despres S, Farioli-Vecchioli S, Bonfanti L. Ghibaudi M, et al. Front Cell Neurosci. 2023 Jul 28;17:1205173. doi: 10.3389/fncel.2023.1205173. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37576566 Free PMC article. - Citalopram Administration Does Not Promote Function or Histological Recovery after Spinal Cord Injury.
Lima R, Monteiro S, Gomes ED, Vasconcelos NL, Assunção-Silva R, Morais M, Salgado AJ, Silva NA. Lima R, et al. Int J Mol Sci. 2020 Jul 17;21(14):5062. doi: 10.3390/ijms21145062. Int J Mol Sci. 2020. PMID: 32709070 Free PMC article.
References
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. - PubMed
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. - PubMed
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases