Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions - PubMed (original) (raw)
Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions
Lassi Liljeroos et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Measles virus is a highly infectious, enveloped, pleomorphic virus. We combined electron cryotomography with subvolume averaging and immunosorbent electron microscopy to characterize the 3D ultrastructure of the virion. We show that the matrix protein forms helices coating the helical ribonucleocapsid rather than coating the inner leaflet of the membrane, as previously thought. The ribonucleocapsid is folded into tight bundles through matrix-matrix interactions. The implications for virus assembly are that the matrix already tightly interacts with the ribonucleocapsid in the cytoplasm, providing a structural basis for the previously observed regulation of RNA transcription by the matrix protein. Next, the matrix-covered ribonucleocapsids are transported to the plasma membrane, where the matrix interacts with the envelope glycoproteins during budding. These results are relevant to the nucleocapsid organization and budding of other paramyxoviruses, where isolated matrix has been observed to form helices.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
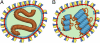
Schematic diagram illustrating two possible ultrastructural models for MV. (A) M coats the viral membrane and the nucleocapsid is free in the interior. (B) M coats the helical nucleocapsid. Color key: nucleocapsid, brown; M, light blue; membrane, red; H, dark blue; F, yellow.
Fig. 2.
MV ultrastructure and nucleocapsid organization. (A–D) Tomographic slices of MV showing the general morphology of the virions. In the virions, two types of tubular structures with 20-nm (white arrow) and 30-nm (black arrows) diameters can be identified. See corresponding
Movies S1
,
S2
,
S3
, and
S4
. (C) A vesicle inside a virion is indicated with an asterisk. (E–G) Different types of nucleocapsid structures from broken virions are shown: a 30-nm structure in E, a partially-covered 20-nm structure in F, and a completely bare 20-nm structure in G. Tomographic slices are 7.7-nm thick. (Scale bar, 100 nm.)
Fig. 3.
Membrane density profile. (A) A slab of density is shown for a tomographic reconstruction of one virion. Part of the membrane density defined for the analysis is indicated as a gray surface. Some surface normals used for extracting and orienting subvolumes are shown as sticks. (B) Plot of density distribution calculated from the extracted subvolumes as a function of distance from the center of the membrane. The extent of the membrane and glycoprotein layer (F/H) is indicated with bars.
Fig. 4.
Electron micrographs of MV-infected and m and n cotransfected cell lysates prepared by immunosorbent EM. (A and B) Three different tubular forms can be observed from infected cells: (A, anti-N grid) 20-nm nucleocapsids with two different packing modes marked with a black arrow [similar to intact recombinant nucleocapsids (–24)] and a white arrow [similar to trypsin-treated recombinant nucleocapsids (–24)] and (B, anti-M grid) 30-nm tubes where matrix covers the nucleocapsids. In C a hollow 30-nm tube from an m and n cotransfected cell lysate (anti-M grid) is shown together with a 20-nm nucleocapsid. (Scale bar, 50 nm.)
Fig. 5.
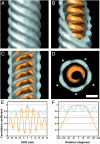
Averaged structure of the MCNC. (A–D) Isosurface representations of the MCNC structure. The structure is seen from the side in A to C, and a slice taken along the axis is shown in D. Both the outer (blue) and inner (orange) parts show a clear helical twist. The transparent surfaces were rendered at a low threshold and the opaque surfaces at a high threshold (0.5 σ and 1.0 σ above the mean density, respectively). (Scale bar, 10 nm.) The stars in D represent the five-start helical arrangement. (E) Translational self-correlation plot shows the correlation coefficient plotted as a function of shift along the helical axis. (F) Rotational self-correlation plot shows the correlation coefficient plotted as a function of rotation around the helical axis (solid line). The same function was plotted for a map correlated against its copy, which had been rotated 180° to turn it upside down (dotted line). The coloring in E and F corresponds to that in A to D.
Fig. 6.
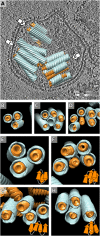
Organization of the MCNC in virions. (A) The averaged structures for the matrix (blue) and nucleocapsid (orange) filaments were placed back into the density map of a virion (one section is shown in gray scale, positive density is black) (
Movie S5
). (B–D) End-on views of the bundles in A are shown from the directions indicated with arrows in A. One M-helix was hollow in C and one NC helix was bare in D, reflecting either inaccuracies in the computational analysis or biological variation in the MCNC structure. (E–H) Different examples of MCNC packing in virions are shown. The embedded schematic diagrams illustrate possible connectivity between the MCNC filaments, consistent with an antiparallel arrangement of neighboring filaments.
Similar articles
- Structure and organization of paramyxovirus particles.
Cox RM, Plemper RK. Cox RM, et al. Curr Opin Virol. 2017 Jun;24:105-114. doi: 10.1016/j.coviro.2017.05.004. Epub 2017 Jun 8. Curr Opin Virol. 2017. PMID: 28601688 Free PMC article. Review. - Structure and assembly of a paramyxovirus matrix protein.
Battisti AJ, Meng G, Winkler DC, McGinnes LW, Plevka P, Steven AC, Morrison TG, Rossmann MG. Battisti AJ, et al. Proc Natl Acad Sci U S A. 2012 Aug 28;109(35):13996-4000. doi: 10.1073/pnas.1210275109. Epub 2012 Aug 13. Proc Natl Acad Sci U S A. 2012. PMID: 22891297 Free PMC article. - Structural virology. Near-atomic cryo-EM structure of the helical measles virus nucleocapsid.
Gutsche I, Desfosses A, Effantin G, Ling WL, Haupt M, Ruigrok RW, Sachse C, Schoehn G. Gutsche I, et al. Science. 2015 May 8;348(6235):704-7. doi: 10.1126/science.aaa5137. Epub 2015 Apr 16. Science. 2015. PMID: 25883315 - Crystal Structure of the Measles Virus Nucleoprotein Core in Complex with an N-Terminal Region of Phosphoprotein.
Guryanov SG, Liljeroos L, Kasaragod P, Kajander T, Butcher SJ. Guryanov SG, et al. J Virol. 2015 Dec 30;90(6):2849-57. doi: 10.1128/JVI.02865-15. J Virol. 2015. PMID: 26719278 Free PMC article. - Molecular mechanism of paramyxovirus budding.
Takimoto T, Portner A. Takimoto T, et al. Virus Res. 2004 Dec;106(2):133-45. doi: 10.1016/j.virusres.2004.08.010. Virus Res. 2004. PMID: 15567493 Review.
Cited by
- Architecture of respiratory syncytial virus revealed by electron cryotomography.
Liljeroos L, Krzyzaniak MA, Helenius A, Butcher SJ. Liljeroos L, et al. Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):11133-8. doi: 10.1073/pnas.1309070110. Epub 2013 Jun 17. Proc Natl Acad Sci U S A. 2013. PMID: 23776214 Free PMC article. - Receptor-Targeted Nipah Virus Glycoproteins Improve Cell-Type Selective Gene Delivery and Reveal a Preference for Membrane-Proximal Cell Attachment.
Bender RR, Muth A, Schneider IC, Friedel T, Hartmann J, Plückthun A, Maisner A, Buchholz CJ. Bender RR, et al. PLoS Pathog. 2016 Jun 9;12(6):e1005641. doi: 10.1371/journal.ppat.1005641. eCollection 2016 Jun. PLoS Pathog. 2016. PMID: 27281338 Free PMC article. - Structure and assembly of double-headed Sendai virus nucleocapsids.
Zhang N, Shan H, Liu M, Li T, Luo R, Yang L, Qi L, Chu X, Su X, Wang R, Liu Y, Sun W, Shen QT. Zhang N, et al. Commun Biol. 2021 Apr 22;4(1):494. doi: 10.1038/s42003-021-02027-y. Commun Biol. 2021. PMID: 33888861 Free PMC article. - Measles Virus Ribonucleoprotein Complexes Rapidly Spread across Well-Differentiated Primary Human Airway Epithelial Cells along F-Actin Rings.
Singh BK, Pfaller CK, Cattaneo R, Sinn PL. Singh BK, et al. mBio. 2019 Nov 26;10(6):e02434-19. doi: 10.1128/mBio.02434-19. mBio. 2019. PMID: 31772054 Free PMC article. - A durable protective immune response to wild-type measles virus infection of macaques is due to viral replication and spread in lymphoid tissues.
Lin WW, Moran E, Adams RJ, Sievers RE, Hauer D, Godin S, Griffin DE. Lin WW, et al. Sci Transl Med. 2020 Apr 1;12(537):eaax7799. doi: 10.1126/scitranslmed.aax7799. Sci Transl Med. 2020. PMID: 32238577 Free PMC article.
References
- Rima BK, Duprex WP. Morbilliviruses and human disease. J Pathol. 2006;208:199–214. - PubMed
- WHO Global reductions in measles mortality 2000–2008 and the risk of measles resurgence. Wkly Epidemiol Rec. 2009;84:509–516. - PubMed
- Yanagi Y, Takeda M, Ohno S. Measles virus: Cellular receptors, tropism and pathogenesis. J Gen Virol. 2006;87:2767–2779. - PubMed
- Riedl P, Moll M, Klenk HD, Maisner A. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 2002;83:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources