Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway - PubMed (original) (raw)
Clinical Trial
Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-κB stress pathway
Kim-Anne Lê et al. Diabetes. 2011 Nov.
Abstract
Objective: To examine in obese young adults the influence of ethnicity and subcutaneous adipose tissue (SAT) inflammation on hepatic fat fraction (HFF), visceral adipose tissue (VAT) deposition, insulin sensitivity (SI), β-cell function, and SAT gene expression.
Research design and methods: SAT biopsies were obtained from 36 obese young adults (20 Hispanics, 16 African Americans) to measure crown-like structures (CLS), reflecting SAT inflammation. SAT, VAT, and HFF were measured by magnetic resonance imaging, and SI and β-cell function (disposition index [DI]) were measured by intravenous glucose tolerance test. SAT gene expression was assessed using Illumina microarrays.
Results: Participants with CLS in SAT (n = 16) were similar to those without CLS in terms of ethnicity, sex, and total body fat. Individuals with CLS had greater VAT (3.7 ± 1.3 vs. 2.6 ± 1.6 L; P = 0.04), HFF (9.9 ± 7.3 vs. 5.8 ± 4.4%; P = 0.03), tumor necrosis factor-α (20.8 ± 4.8 vs. 16.2 ± 5.8 pg/mL; P = 0.01), fasting insulin (20.9 ± 10.6 vs. 9.7 ± 6.6 mU/mL; P < 0.001) and glucose (94.4 ± 9.3 vs. 86.8 ± 5.3 mg/dL; P = 0.005), and lower DI (1,559 ± 984 vs. 2,024 ± 829 × 10(-4) min(-1); P = 0.03). Individuals with CLS in SAT exhibited upregulation of matrix metalloproteinase-9 and monocyte antigen CD14 genes, as well as several other genes belonging to the nuclear factor-κB (NF-κB) stress pathway.
Conclusions: Adipose tissue inflammation was equally distributed between sexes and ethnicities. It was associated with partitioning of fat toward VAT and the liver and altered β-cell function, independent of total adiposity. Several genes belonging to the NF-κB stress pathway were upregulated, suggesting stimulation of proinflammatory mediators.
Trial registration: ClinicalTrials.gov NCT00697580.
Figures
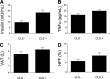
FIG. 1.
Fasting insulin (A), TNF-α concentrations (B), VAT volume (C), and HFF (D) stratified by adipose tissue CLS status. All P values < 0.05.
FIG. 2.
Identification of CD11c+ macrophages (arrows) in CLS− (A and B) and CLS+ (C and D) individuals. Note the presence of CD11c+ immunoreactivity (brown staining) only in subjects with CLS. (A high-quality digital representation of this figure is available in the online issue.)
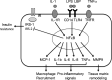
FIG. 3.
In individuals with high macrophage aggregation around dead adipocytes as CLS, activation stimuli such as IL-1, lipopolysaccharide (LPS) and lipopolysaccharide binding protein (LBP), and TNF-α bind to their appropriate membrane receptors. Transduction of signal from receptors activates the NF-κB pathway, which results in transcription of several secreted molecules and downregulates insulin signaling. In adipocytes, MCP-1 recruits macrophages and MMP9 stimulates adipocyte enlargement, whereas secreted ILs and TNF-α further activate inflammatory cascades and participate to insulin resistance development. TLR, Toll-like receptor; TNFR, TNF receptor.
Similar articles
- A Role of the Inflammasome in the Low Storage Capacity of the Abdominal Subcutaneous Adipose Tissue in Obese Adolescents.
Kursawe R, Dixit VD, Scherer PE, Santoro N, Narayan D, Gordillo R, Giannini C, Lopez X, Pierpont B, Nouws J, Shulman GI, Caprio S. Kursawe R, et al. Diabetes. 2016 Mar;65(3):610-8. doi: 10.2337/db15-1478. Epub 2015 Dec 30. Diabetes. 2016. PMID: 26718495 Free PMC article. - Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults.
Neeland IJ, Ayers CR, Rohatgi AK, Turer AT, Berry JD, Das SR, Vega GL, Khera A, McGuire DK, Grundy SM, de Lemos JA. Neeland IJ, et al. Obesity (Silver Spring). 2013 Sep;21(9):E439-47. doi: 10.1002/oby.20135. Epub 2013 May 19. Obesity (Silver Spring). 2013. PMID: 23687099 Free PMC article. - Adipose tissue 11βHSD1 gene expression, βcell function and ectopic fat in obese African Americans versus Hispanics.
Gyllenhammer LE, Alderete TL, Mahurka S, Allayee H, Goran MI. Gyllenhammer LE, et al. Obesity (Silver Spring). 2014 Jan;22(1):14-8. doi: 10.1002/oby.20571. Epub 2013 Sep 10. Obesity (Silver Spring). 2014. PMID: 23836520 - Visceral adiposity and inflammatory bowel disease.
Rowan CR, McManus J, Boland K, O'Toole A. Rowan CR, et al. Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review. - Under the Surface of Subcutaneous Adipose Tissue Biology.
Pandžić Jakšić V, Grizelj D. Pandžić Jakšić V, et al. Acta Dermatovenerol Croat. 2016 Dec;24(4):250-260. Acta Dermatovenerol Croat. 2016. PMID: 28128075 Review.
Cited by
- Targeting adipose tissue inflammation to treat the underlying basis of the metabolic complications of obesity.
Goran MI, Alderete TL. Goran MI, et al. Nestle Nutr Inst Workshop Ser. 2012;73:49-60; discussion p61-6. doi: 10.1159/000341287. Epub 2012 Oct 29. Nestle Nutr Inst Workshop Ser. 2012. PMID: 23128765 Free PMC article. Review. - Visceral adiposopathy: a vascular perspective.
Farb MG, Gokce N. Farb MG, et al. Horm Mol Biol Clin Investig. 2015 Feb;21(2):125-36. doi: 10.1515/hmbci-2014-0047. Horm Mol Biol Clin Investig. 2015. PMID: 25781557 Free PMC article. Review. - SBP2 deficiency in adipose tissue macrophages drives insulin resistance in obesity.
Wang N, Tan HY, Li S, Wang D, Xu Y, Zhang C, Xia W, Che CM, Feng Y. Wang N, et al. Sci Adv. 2019 Aug 14;5(8):eaav0198. doi: 10.1126/sciadv.aav0198. eCollection 2019 Aug. Sci Adv. 2019. PMID: 31453320 Free PMC article. - An integrative epi-transcriptomic approach identifies the human cartilage chitinase 3-like protein 2 (CHI3L2) as a potential mediator of B12 deficiency in adipocytes.
Ogunkolade BW, Adaikalakoteswari A, Cardoso SR, Lowe R, Patel N, Rakyan V, Finer S, Wabitsch M, Saravanan P, Tripathi G, Bochukova E, Hitman GA. Ogunkolade BW, et al. Epigenetics. 2022 Oct;17(10):1219-1233. doi: 10.1080/15592294.2021.2003043. Epub 2021 Nov 25. Epigenetics. 2022. PMID: 34818986 Free PMC article. - Beneficial Short-Term Effects of Bariatric Surgery on Nutritional Inflammatory Profile and Metabolic Biomarkers.
Seva DC, Mônico-Neto M, Antunes HKM, Pino JMV, Bittencourt LRA, Galvão TD, Dâmaso AR, Oyama LM, Shivappa N, Hébert JR, Tufik S, da Silveira Campos RM. Seva DC, et al. Obes Surg. 2023 Sep;33(9):2789-2798. doi: 10.1007/s11695-023-06743-8. Epub 2023 Aug 4. Obes Surg. 2023. PMID: 37540480
References
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010;72:219–246 - PubMed
- Kolak M, Westerbacka J, Velagapudi VR, et al. . Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes 2007;56:1960–1968 - PubMed
- Stefan N, Kantartzis K, Machann J, et al. . Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008;168:1609–1616 - PubMed
- Karelis AD, Faraj M, Bastard JP, et al. . The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005;90:4145–4150 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous