Synaptic vesicle exocytosis - PubMed (original) (raw)
Review
Synaptic vesicle exocytosis
Thomas C Südhof et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
Presynaptic nerve terminals release neurotransmitters by synaptic vesicle exocytosis. Membrane fusion mediating synaptic exocytosis and other intracellular membrane traffic is affected by a universal machinery that includes SNARE (for "soluble NSF-attachment protein receptor") and SM (for "Sec1/Munc18-like") proteins. During fusion, vesicular and target SNARE proteins assemble into an α-helical trans-SNARE complex that forces the two membranes tightly together, and SM proteins likely wrap around assembling trans-SNARE complexes to catalyze membrane fusion. After fusion, SNARE complexes are dissociated by the ATPase NSF (for "N-ethylmaleimide sensitive factor"). Fusion-competent conformations of SNARE proteins are maintained by chaperone complexes composed of CSPα, Hsc70, and SGT, and by nonenzymatically acting synuclein chaperones; dysfunction of these chaperones results in neurodegeneration. The synaptic membrane-fusion machinery is controlled by synaptotagmin, and additionally regulated by a presynaptic protein matrix (the "active zone") that includes Munc13 and RIM proteins as central components.
Figures
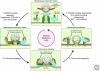
Figure 1.
The synaptic vesicle cycle. A presynaptic nerve terminal is depicted schematically as it contacts a postsynaptic neuron. The synaptic vesicle cycle consists of exocytosis (red arrows) followed by endocytosis and recycling (yellow arrows). Synaptic vesicles (green circles) are filled with neurotransmitters (NT; red dots) by active transport (neurotransmitter uptake) fueled by an electrochemical gradient established by a proton pump that acidifies the vesicle interior (vesicle acidification; green background). In preparation to synaptic exocytosis, synaptic vesicles are docked at the active zone, and primed by an ATP-dependent process that renders the vesicles competent to respond to a Ca2+-signal. When an action potential depolarizes the presynaptic membrane, Ca2+-channels open, causing a local increase in intracellular Ca2+ at the active zone that triggers completion of the fusion reaction. Released neurotransmitters then bind to receptors associated with the postsynaptic density (PSD). After fusion pore opening, synaptic vesicles probably recycle via three alternative pathways: local refilling with neurotransmitters without undocking (“kiss-and-stay”), local recycling with undocking (“kiss-and-run”), and full recycling of vesicles with passage through an endosomal intermediate. (Adapted from Südhof 2004.)
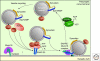
Figure 2.
The SNARE/SM protein cycle. The diagram on top depicts SNARE and SM proteins prior to fusion, when they are localized to the membranes either as natively unfolded proteins (e.g., the R-SNARE synaptobrevin/VAMP) or as proteins folded in complexes distinct from canonical SNARE complexes (e.g., binding of the SM protein Munc18-1 to the closed conformation of syntaxin-1 as shown here, or as syntaxin-1/SNAP-25 heterodimeric complexes). During priming, SNARE proteins partially zipper up into _trans_-complexes, and the SM protein associates with the _trans_-complexes by binding to the syntaxin amino terminus (left diagram). Full SNARE-complex assembly then pulls the membranes apart, opening the fusion pore (bottom diagram), which expands such that the vesicle membrane collapses into the target membrane, and the _trans_-SNARE complexes are converted into _cis_-SNARE complexes (right diagram). Afterward, _cis_-SNARE complexes are dissociated by the ATPase NSF acting in conjunction with its adaptors α/β/γ-SNAPs (no relation to SNAP-25 and its homologs—an unfortunate coincidence of acronyms), and vesicles recycle to start another round of the cycle.
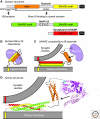
Figure 3.
Structures of synaptic SNARE and SM proteins. (A) Schematic diagram of the domain structures of syntaxin, SNAP-25, and synaptobrevin/VAMP (Habc, Habc-domain; TM, transmembrane region). (B,C) Cartoon of the two modes of interaction of the SM protein Munc18 with SNARE proteins during synaptic exocytosis: Binding of Munc18 to the closed conformation of syntaxin-1 that occludes the SNARE motif (B), and binding of Munc18 to assembling SNARE _trans_-complexes that depends on the syntaxin-1 amino terminus (C). Note that the precise mode of Munc18 binding to assembling SNARE complexes is unknown, apart from the fact that it is anchored by interaction of the syntaxin amino terminus (indicated by an N) with the N-lobe of Munc18; the arrow indicates the uncertain atomic nature of this binding, which may involve wrapping of Munc18 around the SNARE helical bundle analogous to the binding of Munc18 to the closed conformation of syntaxin (Südhof and Rothman 2009). (i) Atomic structures of the fully assembled SNARE complex, the synaxin-1A Habc domain, and Munc18 containing a bound syntaxin amino-terminal peptide (blue), drawn to scale. (Data for structures are from Sutton et al. 1998, Fernandez et al. 1998, and Hu et al. 2011, respectively.) Arrow indicates uncertainty in how precisely Munc18 binds to SNARE complexes apart from the interaction of the syntaxin amino terminus with the Munc18 N-lobe.
Figure 4.
Two types of chaperones support SNARE protein function. Synaptic SNARE proteins are subject to continuous folding and unfolding reactions, creating the potential for misfolding that is counteracted by at least two SNARE chaperones implicated in neurodegeneration: the classical chaperone complex containing CSPα (for cysteine-string protein α), Hsc70, and SGT (red shapes), and the nonclassical chaperones α/β/γ-synucleins (yellow shapes). CSPα and synucleins are vesicle proteins that act by distinct mechanisms. CSPα forms a complex with Hsc70 and SGT on the vesicle that binds to SNAP-25 on the target membrane and supports the functional competence of SNAP-25 to engage in SNARE complexes, whereas synucleins are bound to phospholipids and synaptobrevin (Syb)/VAMP on the vesicles, and bind to assembling SNARE complexes to support their folding.
Figure 5.
Model of active zone protein function. A complex composed of four out of the six canonical components of active zones (Munc13, α-liprins, RIMs, and RIM-BPs) is shown; the remaining two canonical active zone proteins (ELKS and Piccolo/Bassoon) bind peripherally to the complex, and are not shown. The active zone complex is formed by an interaction of the amino-terminal zinc-finger domain of RIM with the amino-terminal C2A-domain of Munc13; by an interaction of a proline-rich sequence in RIM with a RIM-BP SH3-domain, and by a binding of α-liprins to the RIM C2B-domain. The complex is anchored on synaptic vesicles via RIM binding to Rab3 and possibly also via RIM binding synaptotagmin (not shown), and on the plasma membrane via direct binding of RIM and of RIM-BP to N- and P/Q-type Ca2+-channels. Note that there are likely additional interactions among the various active zone proteins, and between these proteins and the presynaptic plasma membrane. Ca2+-ions are shown as dots; most of the active zone proteins contain C2-domains similar to synaptotagmin, but only a subset of the C2-domains bind Ca2+.
Similar articles
- Membrane fusion: grappling with SNARE and SM proteins.
Südhof TC, Rothman JE. Südhof TC, et al. Science. 2009 Jan 23;323(5913):474-7. doi: 10.1126/science.1161748. Science. 2009. PMID: 19164740 Free PMC article. Review. - The molecular machinery of synaptic vesicle exocytosis.
Li L, Chin LS. Li L, et al. Cell Mol Life Sci. 2003 May;60(5):942-60. doi: 10.1007/s00018-003-2240-7. Cell Mol Life Sci. 2003. PMID: 12827282 Free PMC article. Review. - Role of C2 domain proteins during synaptic vesicle exocytosis.
Martens S. Martens S. Biochem Soc Trans. 2010 Feb;38(Pt 1):213-6. doi: 10.1042/BST0380213. Biochem Soc Trans. 2010. PMID: 20074062 - [Molecular mechanisms of SNARE-mediated synaptic vesicle exocytosis].
Takamori S. Takamori S. Tanpakushitsu Kakusan Koso. 2008 Dec;53(16 Suppl):2078-83. Tanpakushitsu Kakusan Koso. 2008. PMID: 21038588 Review. Japanese. No abstract available. - The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged?
Rizo J, Südhof TC. Rizo J, et al. Annu Rev Cell Dev Biol. 2012;28:279-308. doi: 10.1146/annurev-cellbio-101011-155818. Annu Rev Cell Dev Biol. 2012. PMID: 23057743 Review.
Cited by
- The transforming growth factor beta ligand TIG-2 modulates the function of neuromuscular junction and muscle energy metabolism in Caenorhabditis elegans.
Cheng X, Yan Z, Su Z, Liu J. Cheng X, et al. Front Mol Neurosci. 2022 Oct 28;15:962974. doi: 10.3389/fnmol.2022.962974. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36385772 Free PMC article. - Processive ATP-driven substrate disassembly by the N-ethylmaleimide-sensitive factor (NSF) molecular machine.
Cipriano DJ, Jung J, Vivona S, Fenn TD, Brunger AT, Bryant Z. Cipriano DJ, et al. J Biol Chem. 2013 Aug 9;288(32):23436-45. doi: 10.1074/jbc.M113.476705. Epub 2013 Jun 17. J Biol Chem. 2013. PMID: 23775070 Free PMC article. - Neurotransmitter release: vacuolar ATPase V0 sector c-subunits in possible gene or cell therapies for Parkinson's, Alzheimer's, and psychiatric diseases.
Higashida H, Yokoyama S, Tsuji C, Muramatsu SI. Higashida H, et al. J Physiol Sci. 2017 Jan;67(1):11-17. doi: 10.1007/s12576-016-0462-3. Epub 2016 Jun 11. J Physiol Sci. 2017. PMID: 27289535 Free PMC article. Review. - Alpha-Synuclein is Involved in DYT1 Dystonia Striatal Synaptic Dysfunction.
Ponterio G, Faustini G, El Atiallah I, Sciamanna G, Meringolo M, Tassone A, Imbriani P, Cerri S, Martella G, Bonsi P, Bellucci A, Pisani A. Ponterio G, et al. Mov Disord. 2022 May;37(5):949-961. doi: 10.1002/mds.29024. Epub 2022 Apr 14. Mov Disord. 2022. PMID: 35420219 Free PMC article. - Protein sorting from endosomes to the TGN.
Buser DP, Spang A. Buser DP, et al. Front Cell Dev Biol. 2023 Feb 21;11:1140605. doi: 10.3389/fcell.2023.1140605. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36895788 Free PMC article. Review.
References
- Augustin I, Rosenmund C, Südhof TC, Brose N 1999. Munc-13 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400: 457–461 - PubMed
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R 1993a. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365: 160–163 - PubMed
- Brose N, Hofmann K, Hata Y, Südhof TC 1995. Mammalian homologues of C. elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem 270: 25273–25280 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS053862/NS/NINDS NIH HHS/United States
- P01 NS053862/NS/NINDS NIH HHS/United States
- R01 NS040944/NS/NINDS NIH HHS/United States
- P50 MH086403/MH/NIMH NIH HHS/United States
- MH089054/MH/NIMH NIH HHS/United States
- R01 NS077906/NS/NINDS NIH HHS/United States
- NS37200/NS/NINDS NIH HHS/United States
- MH086403/MH/NIMH NIH HHS/United States
- R01 MH089054/MH/NIMH NIH HHS/United States
- NS40944/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 NS037200/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous