Intracellular nucleic acid sensors and autoimmunity - PubMed (original) (raw)
Review
Intracellular nucleic acid sensors and autoimmunity
Argyrios N Theofilopoulos et al. J Interferon Cytokine Res. 2011 Dec.
Abstract
A collection of molecular sensors has been defined by studies in the last decade that can recognize a diverse array of pathogens and initiate protective immune and inflammatory responses. However, if the molecular signatures recognized are shared by both foreign and self-molecules, as is the case of nucleic acids, then the responses initiated by these sensors may have deleterious consequences. Notably, this adverse occurrence may be of primary importance in autoimmune disease pathogenesis. In this case, microbe-induced damage or mishandled physiologic processes could lead to the generation of microparticles containing self-nucleic acids. These particles may inappropriately gain access to the cytosol or endolysosomes and, hence, engage resident RNA and DNA sensors. Evidence, as reviewed here, strongly indicates that these sensors are primary contributors to autoimmune disease pathogenesis, spearheading efforts toward development of novel therapeutics for these disorders.
Figures
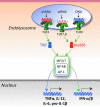
FIG. 1.
Endolysosomal nucleic acid sensors. The presence of nucleic acids in endolysosomes is detected by TLR3 (dsRNA), TLR7 (ssRNA), TLR8 (ssRNA), and TLR9 (DNA). Ligand binding induces TLR dimerization and recruitment of the main signaling adaptors TRIF (used by TLR3) and MyD88 (used by TLRs 7, 8, and 9). These adaptors provide a nucleating structure for the formation of higher-order oligomeric complexes composed of kinases, ubiquitin ligases, and other signaling molecules that mediate activation of transcription factors (IRF3, IRF7, NF-kB, and AP-1) that, on nuclear translocation, promote expression of type I IFNs and other proinflammatory cytokines.
FIG. 2.
TLR compartmentalization and trafficking. Nucleic acid-specific TLRs traffic from the endoplasmic reticulum (ER) to endolysosomes, where they undergo ligand binding and cathepsin-mediated proteolytic cleavage, a process required for efficient signaling. Several proteins have been shown to facilitate TLR translocation to endolysosomes, including the heat-shock protein gp96, PRAT4A, and Unc93b1. Moreover, type I IFN production by pDCs is strictly dependent on TLR localization to specialized compartments termed lysosome-related organelles (LROs). AP-3 (likely together with Unc93b1) mediates TLR translocation to LROs, whereas other molecules in these organelles are required for effective type I IFN induction, including BLOC-1, BLOC-2, and Slc15a4 (a transporter of protons and histidines from the LRO lumen to the cytosol). In addition, the IFN-induced molecule viperin has been shown to localize to lipid bodies and promote assembly of a signaling complex that includes MyD88, IRAK1, and TRAF6, thereby greatly facilitating IRF7 activation and IFN-α production. One study suggests that IRF7 and NF-kB signaling is initiated from LROs and early endosomes, respectively, whereas another study indicates that both pathways are initiated in the LROs.
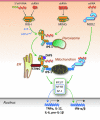
FIG. 3.
Cytosolic RNA sensors. RNA in the cytoplasm is primarily sensed by the helicases RIG-I (RNA with uncapped 5′-triphosphates or short dsRNA) and MDA5 (long dsRNA). Ligand binding induces ATP-dependent conformational changes that allow CARD/CARD homotypic interactions between these helicases and the signaling adaptor IPS-1 localized on either peroxisomes or mitochondria. Engagement of peroxisomal IPS-1 promotes a transient IFN-independent response, whereas at the mitochondrial membrane, the engaged IPS-1 relays a signaling cascade that leads to transcription factor activation and expression of type I IFNs and proinflammatory cytokines. RIG-I signaling also requires the participation of the ER-associated STING and is enhanced by the interaction with ZAPS as well as by TRIM25-mediated K63-linked ubiquitination (U) of CARDs, although unanchored ubiquitin chains also promote RIG-I activation. Another helicase, LGP2, lacks CARDs and appears to facilitate RNA binding to RIG-I and MDA5. In addition, NOD2, a CARD-containing member of the NLR family mostly dedicated to the detection of the peptidoglycan component muramyl dipeptide (MDP) found in both Gram-positive and Gram-negative bacteria, can also recognize ssRNA in the cytosol and promote type I IFN and proinflammatory cytokine production through an IPS-1-mediated pathway.
FIG. 4.
Cytosolic DNA sensors. A large panel of sensors is dedicated to the detection of DNA in the cytosol. Among them, Ku70 induces IFN-λ, DHX9 induces proinflammatory cytokines, and DHX36, DAI, RNA polymerase III, and IFI16 induce production of type I IFNs and proinflammatory cytokines. RNA polymerase III acts by transcribing DNA into RNA molecules bearing uncapped 5′-triphosphates, which bind RIG-I and engage the IPS-1/STING pathway (see Fig. 3). Another sensor, LRRFIP1, binds DNA (or dsRNA) and activates β-catenin, which migrates to the nucleus and potentiates IFNB gene transcription by promoting recruitment of the acetyltransferase p300 to the IFN enhanceosome. In addition, IFI16 and AIM2 induce the assembly of an inflammasome by recruiting pro-caspases via the adaptor ASC, thus leading to caspase-mediated activation and secretion of IL-1β and IL-18. IFI16 (and perhaps other sensors) may recognize DNA in the nucleus and then migrate to the cytoplasm to initiate signal activation.
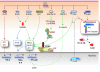
FIG. 5.
Mechanisms by which barriers for TLR recognition of self-nucleic acids are breached in systemic autoimmunity. Various mechanisms usually prevent TLR engagement by self-nucleic acids, including nuclease-mediated degradation, exclusion from endosomes, and methylation. Autoantibodies and BCRs specific for nucleic acid-containing particles (eg, anti-RNP, anti-DNA) or exhibiting rheumatoid factor activity (RF) can overcome these mechanisms, mediating nucleic acid delivery to endosomes in pDCs (via FcγR) and B cells (via BCR), and promoting type I IFN (IFN-α/β) and autoantibody production. Nucleic acid-binding accessory proteins such as HMGB1 (in part via RAGE) and LL37 can also facilitate DNA uptake and TLR engagement. Production of anti-RNP autoantibodies requires TLR7, whereas production of anti-dsDNA and RF autoantibodies requires either TLR7 or TLR9. Thus, the major antigen for autoantibodies to RNP contains RNA (red) and proteins (particle in yellow), whereas the antigenic target of anti-DNA autoantibodies contains DNA (blue), RNA, and other accessory molecules (particle in gray).
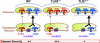
FIG. 6.
Differential Unc93b1-mediated trafficking of TLR7 versus TLR9 may affect lupus pathogenesis in predisposed mice. Increased disease severity in TLR9-deleted lupus-predisposed mice may be due to increased availability of the common Unc93b1 trafficking partner, thus leading to enhanced translocation of the more pathogenic TLR7 to endolysosomes.
Similar articles
- Immune Sensing Mechanisms that Discriminate Self from Altered Self and Foreign Nucleic Acids.
Bartok E, Hartmann G. Bartok E, et al. Immunity. 2020 Jul 14;53(1):54-77. doi: 10.1016/j.immuni.2020.06.014. Immunity. 2020. PMID: 32668228 Free PMC article. Review. - Importance of Nucleic Acid Recognition in Inflammation and Autoimmunity.
Barrat FJ, Elkon KB, Fitzgerald KA. Barrat FJ, et al. Annu Rev Med. 2016;67:323-36. doi: 10.1146/annurev-med-052814-023338. Epub 2015 Nov 2. Annu Rev Med. 2016. PMID: 26526766 Review. - Recognition of nucleic acids by pattern-recognition receptors and its relevance in autoimmunity.
Kawasaki T, Kawai T, Akira S. Kawasaki T, et al. Immunol Rev. 2011 Sep;243(1):61-73. doi: 10.1111/j.1600-065X.2011.01048.x. Immunol Rev. 2011. PMID: 21884167 Free PMC article. Review. - Cytosolic nucleic acid sensors and innate immune regulation.
Ori D, Murase M, Kawai T. Ori D, et al. Int Rev Immunol. 2017 Mar 4;36(2):74-88. doi: 10.1080/08830185.2017.1298749. Epub 2017 Mar 23. Int Rev Immunol. 2017. PMID: 28333574 Review. - Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination.
Jounai N, Kobiyama K, Takeshita F, Ishii KJ. Jounai N, et al. Front Cell Infect Microbiol. 2013 Jan 8;2:168. doi: 10.3389/fcimb.2012.00168. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316484 Free PMC article. Review.
Cited by
- Requirements for innate immune pathways in environmentally induced autoimmunity.
Pollard KM, Kono DH. Pollard KM, et al. BMC Med. 2013 Apr 4;11:100. doi: 10.1186/1741-7015-11-100. BMC Med. 2013. PMID: 23557436 Free PMC article. Review. - Essential requirement for IRF8 and SLC15A4 implicates plasmacytoid dendritic cells in the pathogenesis of lupus.
Baccala R, Gonzalez-Quintial R, Blasius AL, Rimann I, Ozato K, Kono DH, Beutler B, Theofilopoulos AN. Baccala R, et al. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):2940-5. doi: 10.1073/pnas.1222798110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382217 Free PMC article. - Autoreactive B cells in SLE, villains or innocent bystanders?
Hamilton JA, Hsu HC, Mountz JD. Hamilton JA, et al. Immunol Rev. 2019 Nov;292(1):120-138. doi: 10.1111/imr.12815. Epub 2019 Oct 21. Immunol Rev. 2019. PMID: 31631359 Free PMC article. Review. - Human Leukocyte Antigen Genotyping of Idiopathic Subglottic Stenosis.
Rohlfing ML, Hillel AT, Wohler E, Sobreira N, Phillips EJ, Mallal SA, Gelbard A. Rohlfing ML, et al. Laryngoscope. 2023 Oct;133(10):2533-2539. doi: 10.1002/lary.30580. Epub 2023 Feb 2. Laryngoscope. 2023. PMID: 36728247 Free PMC article. - B Cells as a Therapeutic Target in Paediatric Rheumatic Disease.
Wilkinson MGL, Rosser EC. Wilkinson MGL, et al. Front Immunol. 2019 Feb 14;10:214. doi: 10.3389/fimmu.2019.00214. eCollection 2019. Front Immunol. 2019. PMID: 30837988 Free PMC article. Review.
References
- Allam R. Pawar RD. Kulkarni OP. Hornung V. Hartmann G. Segerer S. Akira S. Endres S. Anders HJ. Viral 5'-triphosphate RNA and non-CpG DNA aggravate autoimmunity and lupus nephritis via distinct TLR-independent immune responses. Eur J Immunol. 2008;38(12):3487–3498. - PubMed
- Allen IC. Moore CB. Schneider M. Lei Y. Davis BK. Scull MA. Gris D. Roney KE. Zimmermann AG. Bowzard JB. Ranjan P. Monroe KM. Pickles RJ. Sambhara S. Ting JP. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34(6):854–865. - PMC - PubMed
- Ananieva O. Darragh J. Johansen C. Carr JM. McIlrath J. Park JM. Wingate A. Monk CE. Toth R. Santos SG. Iversen L. Arthur JS. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9(9):1028–1036. - PubMed
- Andersson U. Rauvala H. Introduction: HMGB1 in inflammation and innate immunity. J Intern Med. 2011;270(4):296–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR053731/AR/NIAMS NIH HHS/United States
- AR30103/AR/NIAMS NIH HHS/United States
- AR39555/AR/NIAMS NIH HHS/United States
- AR053228/AR/NIAMS NIH HHS/United States
- R37 AR039555/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources