Twisted tango: brain tumor neurovascular interactions - PubMed (original) (raw)
Review
. 2011 Oct 26;14(11):1375-81.
doi: 10.1038/nn.2955.
Affiliations
- PMID: 22030548
- PMCID: PMC3615423
- DOI: 10.1038/nn.2955
Review
Twisted tango: brain tumor neurovascular interactions
Anita B Hjelmeland et al. Nat Neurosci. 2011.
Abstract
The brain is a complicated organ with complexity derived from cellular and microenvironmental interactions. Similarly, brain tumor cells actively modify and are regulated by their microenvironment. Brain tumors are highly heterogeneous and frequently show a cellular hierarchy with self-renewing tumorigenic brain tumor stem cells (BTSCs) at the apex. Although BTSCs are distinct from neural stem cells, they share characteristics, including bidirectional interplay with supportive vasculature critical for maintenance of undifferentiated states and survival. BTSCs stimulate angiogenesis through growth factor secretion and are enriched in perivascular niches. Microenvironmental conditions, including hypoxia, drive expression of stem cell genes and proangiogenic factors, further linking cellular hierarchy regulation and instructive stromal elements. BTSCs may also directly contribute to tumor vasculature through plasticity toward an endothelial lineage. Interrogating the codependence of BTSCs and the perivascular niche may directly inform clinical approaches for brain tumor therapy through targeting of highly angiogenic and tumorigenic cellular subsets.
Figures
Figure 1. Learning the Steps –Isolation and Characterization of Brain Tumor Stem Cells
Brain tumor stem cells (BTSCs) from malignant tumors (glioma, medulloblastoma, ependymoma) can be enriched based on cell surface expression using flow cytometry (markers including but not limited to CD133, A2B5, CD171 (L1CAM), CD15/SSEA1, CD49f (Integrin a6), CD44, and EGFR). Upon enrichment, hierarchy should be validated by functional assays of tumor propagation. BTSCs often have cellular phenotypes associated with the promotion of angiogenesis, therapeutic resistance, immune evasion, and niche interactions which are elevated in comparison to non-stem tumor cells.
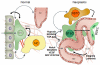
Figure 2. Shall we dance? Coordinated communication between cells in the perivascular niche
Neural stem cells (NSCs) present in the perivascular niche rely on a series of signals between the extracellular matrix (ECM), blood vessels (BV), ependymal cells (E), and other niche cells (NC) to promote their maintenance. Brain tumor stem cells (BTSCs) rely on similar interactions within the perivascular niche, which also consist of ECM, non-stem tumor cells (NSTC), and tumor blood vessels (TBV). BTSC plasticity towards an endothelial lineage and incorporation of these BTSCs derived endothelial cells (BDECs) into the vasculature may also contribute to the perivascular niche. Figure modified from Gilbertson & Rich.
Similar articles
- [Progress in the study of brain tumor stem cells as treatment targets].
Hide T, Kuratsu J. Hide T, et al. Brain Nerve. 2009 Jul;61(7):781-9. Brain Nerve. 2009. PMID: 19618855 Review. Japanese. - Microenvironment and brain tumor stem cell maintenance: impact of the niche.
Herold-Mende C, Mock A. Herold-Mende C, et al. Anticancer Agents Med Chem. 2014;14(8):1065-74. doi: 10.2174/1871520614666140825103636. Anticancer Agents Med Chem. 2014. PMID: 25175691 Review. - GPD1 Specifically Marks Dormant Glioma Stem Cells with a Distinct Metabolic Profile.
Rusu P, Shao C, Neuerburg A, Acikgöz AA, Wu Y, Zou P, Phapale P, Shankar TS, Döring K, Dettling S, Körkel-Qu H, Bekki G, Costa B, Guo T, Friesen O, Schlotter M, Heikenwalder M, Tschaharganeh DF, Bukau B, Kramer G, Angel P, Herold-Mende C, Radlwimmer B, Liu HK. Rusu P, et al. Cell Stem Cell. 2019 Aug 1;25(2):241-257.e8. doi: 10.1016/j.stem.2019.06.004. Epub 2019 Jul 11. Cell Stem Cell. 2019. PMID: 31303549 - Brain tumor stem cells.
Xie Z. Xie Z. Neurochem Res. 2009 Dec;34(12):2055-66. doi: 10.1007/s11064-009-0079-5. Epub 2009 Oct 24. Neurochem Res. 2009. PMID: 19856100 Review. - Deadly teamwork: neural cancer stem cells and the tumor microenvironment.
Lathia JD, Heddleston JM, Venere M, Rich JN. Lathia JD, et al. Cell Stem Cell. 2011 May 6;8(5):482-5. doi: 10.1016/j.stem.2011.04.013. Cell Stem Cell. 2011. PMID: 21549324 Free PMC article. Review.
Cited by
- Estimation of intraoperative brain shift by combination of stereovision and doppler ultrasound: phantom and animal model study.
Mohammadi A, Ahmadian A, Azar AD, Sheykh AD, Amiri F, Alirezaie J. Mohammadi A, et al. Int J Comput Assist Radiol Surg. 2015 Nov;10(11):1753-64. doi: 10.1007/s11548-015-1216-z. Epub 2015 May 10. Int J Comput Assist Radiol Surg. 2015. PMID: 25958061 - Multifunctional nanocarriers for delivering siRNA and miRNA in glioblastoma therapy: advances in nanobiotechnology-based cancer therapy.
Shetty K, Yasaswi S, Dutt S, Yadav KS. Shetty K, et al. 3 Biotech. 2022 Nov;12(11):301. doi: 10.1007/s13205-022-03365-2. Epub 2022 Sep 30. 3 Biotech. 2022. PMID: 36276454 Free PMC article. Review. - CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry.
Lai CY, Schwartz BE, Hsu MY. Lai CY, et al. Cancer Res. 2012 Oct 1;72(19):5111-8. doi: 10.1158/0008-5472.CAN-12-0624. Epub 2012 Aug 3. Cancer Res. 2012. PMID: 22865455 Free PMC article. - DICER governs characteristics of glioma stem cells and the resulting tumors in xenograft mouse models of glioblastoma.
Mansouri S, Singh S, Alamsahebpour A, Burrell K, Li M, Karabork M, Ekinci C, Koch E, Solaroglu I, Chang JT, Wouters B, Aldape K, Zadeh G. Mansouri S, et al. Oncotarget. 2016 Aug 30;7(35):56431-56446. doi: 10.18632/oncotarget.10570. Oncotarget. 2016. PMID: 27421140 Free PMC article. - Autophagy in the Immunosuppressive Perivascular Microenvironment of Glioblastoma.
Molina ML, García-Bernal D, Martinez S, Valdor R. Molina ML, et al. Cancers (Basel). 2019 Dec 31;12(1):102. doi: 10.3390/cancers12010102. Cancers (Basel). 2019. PMID: 31906065 Free PMC article. Review.
References
- Stupp R, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
- Phillips HS, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. - PubMed
Publication types
MeSH terms
Grants and funding
- CA142159/CA/NCI NIH HHS/United States
- R01 CA116659/CA/NCI NIH HHS/United States
- CA151522/CA/NCI NIH HHS/United States
- F32 CA142159/CA/NCI NIH HHS/United States
- R01 CA129958/CA/NCI NIH HHS/United States
- R01 CA154130/CA/NCI NIH HHS/United States
- CA129958/CA/NCI NIH HHS/United States
- R01 CA151522/CA/NCI NIH HHS/United States
- CA154130/CA/NCI NIH HHS/United States
- CA116659/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous