Chk2 deficiency in Myc overexpressing lymphoma cells elicits a synergistic lethal response in combination with PARP inhibition - PubMed (original) (raw)
Chk2 deficiency in Myc overexpressing lymphoma cells elicits a synergistic lethal response in combination with PARP inhibition
Andreas Höglund et al. Cell Cycle. 2011.
Abstract
Myc is a transcription factor frequently found deregulated in human cancer. The Myc-mediated cellular transformation process is associated with fast proliferative cells and inherent genomic instability, giving rise to malignant, invasive neoplasms with poor prognosis for survival. Transcription-independent functions of Myc include stimulation of replication. Excessive Myc expression stimulates a replication-associated DNA damage response that signals via the phosphoinositide-3-kinase (PI3K)-related protein kinases (PIKKs) ATM and ATR. These, in turn, activate the DNA damage transducers Chk1 and Chk2. Here, we show that Myc can stimulate Chek2 transcript indirectly in vitro as well as in B cells of λ-Myc transgenic mice or in the intestine of Apc (Min) mice. However, Chk2 is dispensable for Myc's ability to transform cells in vitro and for the survival of established lymphoma cells from λ-Myc transgenic mice. Chk2 deficiency induces polyploidy and slow growth, but the cells are viable and protected against DNA damage. Furthermore, inhibition of both Chk1/Chk2 with AZD7762 induces cell death and significantly delays disease progression of transplanted lymphoma cells in vivo. DNA damage recruits PARP family members to sites of DNA breaks that, in turn, facilitate the induction of DNA repair. Strikingly, combining Chk2 and PARP inhibition elicits a synergistic lethal response in the context of Myc overexpression. Our data indicates that only certain types of chemotherapy would give rise to a synergistic lethal response in combination with specific Chk2 inhibitors, which will be important if Chk2 inhibitors enter the clinic.
Figures
Figure 1
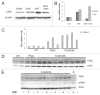
Myc upregulates Chk2 mRNA and protein levels. (A) Protein gel blot analysis of NIH 3T3 fibroblasts infected with MSCV-MycER-IRES-puro retrovirus. The nuclear translocation of MycER was induced by 4-HT for 24 h. Whole-cell lysates were harvested and analyzed using antibodies directed against the indicated proteins. (B) qRT-PCR analysis of Chek2 and Odc transcript levels in _p53_-knockout mEFs infected with MSCV-MycER-IRES-GFP retrovirus. 4-HT was added to the cells, and transcript expression was measured 24 h later, in the presence or absence of 1 µg/ml cycloheximide (CHX) in the growth media. (C) qRT-PCR analysis of Chek2 transcript levels in (B) cells from WT and γ-Myc mice as well as tumors developed in the γ-Myc transgenic animals. (D) Protein gel blot analysis of Chk2 protein levels in < 6-week-old wild-type (WT) and pre-cancerous γ-Myc mice compared with palpable lymphomas harvested from sick animals. (E) Lymphomas from sick γ-Myc mice were either treated with FastAP™ alkaline phosphatase or mock-treated (heat-inactivated alkaline phosphatase) and then analyzed by protein gel blot.
Figure 2
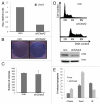
Chk2 is dispensable for Myc-induced colony formation, and Chk2 deficiency protects lymphoma cells against DNA damage. (A) qRT-PCR analysis of Chek2 transcript levels NIH-3T3 fibroblasts infected with MSCV-Myc-IRES-GFP retroviruses and shRNA against Chek2 or a non-target shRNA. (B) Clonogenic survival assay of 104 NIH-3T3 fibroblasts of cells in (A). The cells were grown to confluence during the course of 10 d and then stained with Commasie blue. (C) The cells constructed in (A) were also put in soft agar and grown for two weeks. Colonies were counted using bright-field microscopy on three separate plates, here presented as an average of these. (D) A mouse lymphoma cell line from the γ-Myc transgenic mouse was infected with shRNA against Chek2 or control vector (protein gel blot inset). After a few passages, the cells were harvested, stained with propidium iodine (PI) and analyzed by flow cytometry to generate DNA histograms. (E) The Chk2-deficient cells were treated for 48 h with 1 µM Chekin, 50 nM Taxol or 5 Gy of gamma irradiation (IR). Apoptosis was scored by analysis of the sub-G1 population of PI stained cells using flow cytometry.
Figure 3
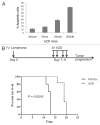
Chk1/Chk2 inhibition induces apoptosis in mouse lymphoma cells and delays disease onset in vivo. (A) Lymphoma cells from the γ-Myc transgenic mouse were treated with increasingly higher doses of AZD7762 (AZD) during the course of 48 h as indicated. Apoptosis was scored by analysis of the sub-G1 population of PI-stained cells using flow cytometry. (B) Recipient mice were transplanted with B-cell lymphoma cells and, after a week, treated (25 mg/kg/qd) for 4 d with IV injections of AZD (dose schedule insert). The mice were then monitored for palpable lymphomas. AZD-treated animals had a median survival time of 19 d compared with 13 d for vehicle-treated animals (p = 0.0016).
Figure 4
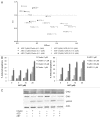
Dual Chk2 and PARP inhibition elicits a synergistic lethal response. (A) Lymphoma cells from the γ-Myc transgenic mouse were treated with combinations of either AZD and ABT-888 (ABT) or Chekin and ABT and assessed for apoptosis by analysis of the sub-G1 population of PI-stained cells using flow cytometry. Synergistic response was calculated using the median effect analysis of the CalcuSyn software (Biosoft). Depicted are also drug doses of Chekin, ABT and AZD used (µM). [Combination Index (CI) values of < 1 indicate synergy; effect describes the fraction of apoptotic cells at the various drug combinations]. (B) Apoptotic response in samples described in (A). AZD and ABT combinatorial treatment leads to an increase in apoptosis with increasing concentrations of the drugs as indicated. (C) Mouse lymphoma cells were treated for 48 h with either Chekin (1 µM) or AZD (0.1 µM) alone or in combinations with ABT (10 µM) and then harvested for protein gel blot analysis.
Similar articles
- MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells.
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, Yao KT. Wang WJ, et al. Cancer Res. 2013 Feb 1;73(3):1219-31. doi: 10.1158/0008-5472.CAN-12-1408. Epub 2012 Dec 26. Cancer Res. 2013. PMID: 23269272 - Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair.
Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, Arumugarajah S, Hylander-Gans L, Morosini D, Simeone DM, Canman CE, Normolle DP, Zabludoff SD, Maybaum J, Lawrence TS. Morgan MA, et al. Cancer Res. 2010 Jun 15;70(12):4972-81. doi: 10.1158/0008-5472.CAN-09-3573. Epub 2010 May 25. Cancer Res. 2010. PMID: 20501833 Free PMC article. - The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer.
Smith J, Tho LM, Xu N, Gillespie DA. Smith J, et al. Adv Cancer Res. 2010;108:73-112. doi: 10.1016/B978-0-12-380888-2.00003-0. Adv Cancer Res. 2010. PMID: 21034966 Review. - Constitutive activation of the DNA damage response pathway as a novel therapeutic target in diffuse large B-cell lymphoma.
Derenzini E, Agostinelli C, Imbrogno E, Iacobucci I, Casadei B, Brighenti E, Righi S, Fuligni F, Ghelli Luserna Di Rorà A, Ferrari A, Martinelli G, Pileri S, Zinzani PL. Derenzini E, et al. Oncotarget. 2015 Mar 30;6(9):6553-69. doi: 10.18632/oncotarget.2720. Oncotarget. 2015. PMID: 25544753 Free PMC article. - Trial Watch: Targeting ATM-CHK2 and ATR-CHK1 pathways for anticancer therapy.
Manic G, Obrist F, Sistigu A, Vitale I. Manic G, et al. Mol Cell Oncol. 2015 Feb 23;2(4):e1012976. doi: 10.1080/23723556.2015.1012976. eCollection 2015 Oct-Dec. Mol Cell Oncol. 2015. PMID: 27308506 Free PMC article. Review.
Cited by
- DNA repair dysregulation from cancer driver to therapeutic target.
Curtin NJ. Curtin NJ. Nat Rev Cancer. 2012 Dec;12(12):801-17. doi: 10.1038/nrc3399. Nat Rev Cancer. 2012. PMID: 23175119 Review. - SGO1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells.
Murakami-Tonami Y, Ikeda H, Yamagishi R, Inayoshi M, Inagaki S, Kishida S, Komata Y, Jan Koster, Takeuchi I, Kondo Y, Maeda T, Sekido Y, Murakami H, Kadomatsu K. Murakami-Tonami Y, et al. Sci Rep. 2016 Aug 19;6:31615. doi: 10.1038/srep31615. Sci Rep. 2016. PMID: 27539729 Free PMC article. - BET bromodomain inhibitors synergize with ATR inhibitors in melanoma.
Muralidharan SV, Einarsdottir BO, Bhadury J, Lindberg MF, Wu J, Campeau E, Bagge RO, Stierner U, Ny L, Nilsson LM, Nilsson JA. Muralidharan SV, et al. Cell Death Dis. 2017 Aug 10;8(8):e2982. doi: 10.1038/cddis.2017.383. Cell Death Dis. 2017. PMID: 28796244 Free PMC article. - DDRugging glioblastoma: understanding and targeting the DNA damage response to improve future therapies.
Rominiyi O, Collis SJ. Rominiyi O, et al. Mol Oncol. 2022 Jan;16(1):11-41. doi: 10.1002/1878-0261.13020. Epub 2021 Jun 11. Mol Oncol. 2022. PMID: 34036721 Free PMC article. Review. - Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance.
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G, Mazzarino MC, Fagone P, Nicoletti F, Bäsecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Chiarini F, Evangelisti C, Cocco L, Martelli AM. McCubrey JA, et al. Oncotarget. 2012 Oct;3(10):1068-111. doi: 10.18632/oncotarget.659. Oncotarget. 2012. PMID: 23085539 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous