Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data - PubMed (original) (raw)
doi: 10.1002/gcc.20937. Epub 2011 Oct 27.
Toru Motoi, Raya Khanin, Adam Olshen, Fredrik Mertens, Julia Bridge, Paola Dal Cin, Cristina R Antonescu, Samuel Singer, Meera Hameed, Judith V M G Bovee, Pancras C W Hogendoorn, Nicholas Socci, Marc Ladanyi
Affiliations
- PMID: 22034177
- PMCID: PMC3235801
- DOI: 10.1002/gcc.20937
Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data
Lu Wang et al. Genes Chromosomes Cancer. 2012 Feb.
Abstract
Cancer gene fusions that encode a chimeric protein are often characterized by an intragenic discontinuity in the RNA\expression levels of the exons that are 5' or 3' to the fusion point in one or both of the fusion partners due to differences in the levels of activation of their respective promoters. Based on this, we developed an unbiased, genome-wide bioinformatic screen for gene fusions using Affymetrix Exon array expression data. Using a training set of 46 samples with different known gene fusions, we developed a data analysis pipeline, the "Fusion Score (FS) model", to score and rank genes for intragenic changes in expression. In a separate discovery set of 41 tumor samples with possible unknown gene fusions, the FS model generated a list of 552 candidate genes. The transcription factor gene NCOA2 was one of the candidates identified in a mesenchymal chondrosarcoma. A novel HEY1-NCOA2 fusion was identified by 5' RACE, representing an in-frame fusion of HEY1 exon 4 to NCOA2 exon 13. RT-PCR or FISH evidence of this HEY1-NCOA2 fusion was present in all additional mesenchymal chondrosarcomas tested with a definitive histologic diagnosis and adequate material for analysis (n = 9) but was absent in 15 samples of other subtypes of chondrosarcomas. We also identified a NUP107-LGR5 fusion in a dedifferentiated liposarcoma but analysis of 17 additional samples did not confirm it as a recurrent event in this sarcoma type. The novel HEY1-NCOA2 fusion appears to be the defining and diagnostic gene fusion in mesenchymal chondrosarcomas.
Copyright © 2011 Wiley-Liss, Inc.
Figures
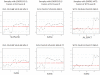
Figure 1
Representative exon expression plots of samples with known fusions for validation of the data analysis pipeline. To visualize the exon level expression data, the normalized relative expression level of each exon (Y axis) was plotted along exons of a given gene from 5′ to 3′ (X axis). Red lines indicated possible exon clusters of the given gene, and solid black triangle indicated each corresponding probe set. Four plots on the left were FLI1 exon expression plots in four different samples with EWSR1-FLI1 fusion. Tumor cell line SK-PN-DW (upper left) and patient sample ES2 (lower left) had EWSR1-FLI1 type I fusion (fusion at FLI1 exon 6); Patient samples ES4 (upper middle) and ES6 (lower middle) had EWSR1-FLI1 type II fusion (fusion at FLI1 exon 5). Two plots on the right were WT1 exon expression plots in two different samples with EWSR1-WT1 fusion. Tumor cell line JN-DSRCT (upper right) and patient sample DS2 (lower right) both show evidence of fusion at WT1 exon 8.
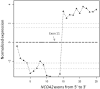
Figure 2
Exon expression plot of NCOA2 in the mesenchymal chondrosarcoma sample UF5, showing the intragenic change in NCOA2 expression. The change point was mapped between exon 12 and exon 13. This exon expression pattern was not seen in any other tumor samples or in normal control tissues.
Figure 3
Identification of the novel HEY1-NCOA2 fusion in the mesenchymal chondrosarcoma case UF5. A, Upper: partial schema of HEY1 and NCOA2 gene structures in relation to the formation of HEY1-NCOA2 fusion transcript; Lower: Partial sequence of the HEY1-NCOA2 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of HEY1 exon 4 to NCOA2 exon 13. B, RT-PCR detection of HEY1-NCOA2 fusion. The presence of HEY1-NCOA2 fusion transcript was confirmed by RT-PCR using two independent sets of HEY1 forward and NCOA2 reverse primers, HEY1-F1/NCOA2-R3 and HEY1-F2/NCOA2-R2. Sequencing of bands a and b confirmed the HEY1-NCOA2 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA). C, Schematic diagrams of HEY1, NCOA2 and the predicted HEY1-NCOA2 chimeric proteins as well as mapping of NCOA2 breakpoints of four different fusions in protein level. Symbols and abbreviations for the diagram of HEY1: blue bar stands for the basic motif; HLH, helix-loop-helix domain. Symbols and abbreviations for the diagram of NCOA2: bHLH, basic helix-loop-helix domain; PAS, Per/ARN/Sim homologous domain; NID, nuclear receptor interaction domain; AD1/CID, transcriptional activation domain 1/CBP/p300 interaction domain; AD2, transcriptional activation domain 2; Q, glutamine rich region; red bars stand for LXXLL motifs; orange bar indicates the LLXXLXXXL motif.
Figure 3
Identification of the novel HEY1-NCOA2 fusion in the mesenchymal chondrosarcoma case UF5. A, Upper: partial schema of HEY1 and NCOA2 gene structures in relation to the formation of HEY1-NCOA2 fusion transcript; Lower: Partial sequence of the HEY1-NCOA2 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of HEY1 exon 4 to NCOA2 exon 13. B, RT-PCR detection of HEY1-NCOA2 fusion. The presence of HEY1-NCOA2 fusion transcript was confirmed by RT-PCR using two independent sets of HEY1 forward and NCOA2 reverse primers, HEY1-F1/NCOA2-R3 and HEY1-F2/NCOA2-R2. Sequencing of bands a and b confirmed the HEY1-NCOA2 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA). C, Schematic diagrams of HEY1, NCOA2 and the predicted HEY1-NCOA2 chimeric proteins as well as mapping of NCOA2 breakpoints of four different fusions in protein level. Symbols and abbreviations for the diagram of HEY1: blue bar stands for the basic motif; HLH, helix-loop-helix domain. Symbols and abbreviations for the diagram of NCOA2: bHLH, basic helix-loop-helix domain; PAS, Per/ARN/Sim homologous domain; NID, nuclear receptor interaction domain; AD1/CID, transcriptional activation domain 1/CBP/p300 interaction domain; AD2, transcriptional activation domain 2; Q, glutamine rich region; red bars stand for LXXLL motifs; orange bar indicates the LLXXLXXXL motif.
Figure 3
Identification of the novel HEY1-NCOA2 fusion in the mesenchymal chondrosarcoma case UF5. A, Upper: partial schema of HEY1 and NCOA2 gene structures in relation to the formation of HEY1-NCOA2 fusion transcript; Lower: Partial sequence of the HEY1-NCOA2 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of HEY1 exon 4 to NCOA2 exon 13. B, RT-PCR detection of HEY1-NCOA2 fusion. The presence of HEY1-NCOA2 fusion transcript was confirmed by RT-PCR using two independent sets of HEY1 forward and NCOA2 reverse primers, HEY1-F1/NCOA2-R3 and HEY1-F2/NCOA2-R2. Sequencing of bands a and b confirmed the HEY1-NCOA2 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA). C, Schematic diagrams of HEY1, NCOA2 and the predicted HEY1-NCOA2 chimeric proteins as well as mapping of NCOA2 breakpoints of four different fusions in protein level. Symbols and abbreviations for the diagram of HEY1: blue bar stands for the basic motif; HLH, helix-loop-helix domain. Symbols and abbreviations for the diagram of NCOA2: bHLH, basic helix-loop-helix domain; PAS, Per/ARN/Sim homologous domain; NID, nuclear receptor interaction domain; AD1/CID, transcriptional activation domain 1/CBP/p300 interaction domain; AD2, transcriptional activation domain 2; Q, glutamine rich region; red bars stand for LXXLL motifs; orange bar indicates the LLXXLXXXL motif.
Figure 4
RT-PCR detection of the HEY1-NCOA2 fusion in additional mesenchymal chondrosarcoma cases. Upper panel: RT-PCR performed using HEY1 forward primer HEY1-F1 and NCOA2 reverse primer NCOA2-R3. Lower panel: RT-PCR for PGK transcript. MSK_1 to MSK_10 were 10 FFPE samples and L1~L3 were frozen samples. The identical HEY1-NCOA2 fusion transcript was detected in cases MSK_3 (weak positive due to the very poor quality of RNA), MSK_5, MSK_6, L1 and L3. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA).
Figure 5
Interphase FISH detection of HEY1-NCOA2 fusion in mesenchymal chondrosarcomas. A, the schema of FISH-probe design. B, representative HEY1-NCOA2 dual-color FISH pictures. Signal pattern in normal cells was shown in the left panel. In contrast, fused signals (visible as one yellow dot, instead of green dot/red dot side by side) were seen in cells of RT-PCR positive cases MSK_3 (middle) and MSK_6 (right).
Figure 5
Interphase FISH detection of HEY1-NCOA2 fusion in mesenchymal chondrosarcomas. A, the schema of FISH-probe design. B, representative HEY1-NCOA2 dual-color FISH pictures. Signal pattern in normal cells was shown in the left panel. In contrast, fused signals (visible as one yellow dot, instead of green dot/red dot side by side) were seen in cells of RT-PCR positive cases MSK_3 (middle) and MSK_6 (right).
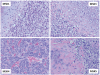
Figure 6
Morphology of four HEY1-NCOA2 positive mesenchymal chondrosarcoma cases showing richly cellular fields of small round or slightly spindled cells and islands of chondroid matrix.
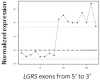
Figure 7
Identification of the novel NUP107-LGR5 fusion in a dedifferentiated liposarcoma case (UF2). A, Exon expression plot of LGR5 in sample UF2, showing the intragenic change in LGR5 expression. The change point was mapped between exon 11 and exon 12. This exon expression pattern was not seen in any other tumor samples or in normal control tissues. B, Upper: The schema of NUP107 and LGR5 partial gene structures as well as the formation of NUP107-LGR5 fusion transcript; Lower: Partial sequence of the NUP107-LGR5 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of NUP107 exon 1 to LGR5 exon 12. C, RT-PCR detection of NUP107-LGR5 fusion. The presence of NUP107-LGR5 fusion transcript was verified by RT-PCR using NUP107 forward and LGR5 reverse primers (NUP107-E1-F, LGR5-E13-RV). Sequencing of the RT-PCR product confirmed the NUP107-LGR5 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA).
Figure 7
Identification of the novel NUP107-LGR5 fusion in a dedifferentiated liposarcoma case (UF2). A, Exon expression plot of LGR5 in sample UF2, showing the intragenic change in LGR5 expression. The change point was mapped between exon 11 and exon 12. This exon expression pattern was not seen in any other tumor samples or in normal control tissues. B, Upper: The schema of NUP107 and LGR5 partial gene structures as well as the formation of NUP107-LGR5 fusion transcript; Lower: Partial sequence of the NUP107-LGR5 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of NUP107 exon 1 to LGR5 exon 12. C, RT-PCR detection of NUP107-LGR5 fusion. The presence of NUP107-LGR5 fusion transcript was verified by RT-PCR using NUP107 forward and LGR5 reverse primers (NUP107-E1-F, LGR5-E13-RV). Sequencing of the RT-PCR product confirmed the NUP107-LGR5 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA).
Figure 7
Identification of the novel NUP107-LGR5 fusion in a dedifferentiated liposarcoma case (UF2). A, Exon expression plot of LGR5 in sample UF2, showing the intragenic change in LGR5 expression. The change point was mapped between exon 11 and exon 12. This exon expression pattern was not seen in any other tumor samples or in normal control tissues. B, Upper: The schema of NUP107 and LGR5 partial gene structures as well as the formation of NUP107-LGR5 fusion transcript; Lower: Partial sequence of the NUP107-LGR5 fusion transcript along with predicted amino acid sequence. The transcript is an in-frame fusion of NUP107 exon 1 to LGR5 exon 12. C, RT-PCR detection of NUP107-LGR5 fusion. The presence of NUP107-LGR5 fusion transcript was verified by RT-PCR using NUP107 forward and LGR5 reverse primers (NUP107-E1-F, LGR5-E13-RV). Sequencing of the RT-PCR product confirmed the NUP107-LGR5 in-frame fusion transcript. Size marker is 100bp DNA ladder (Invitrogen, Carlsbad, CA).
Similar articles
- Genomic profiling identifies genes and pathways dysregulated by HEY1-NCOA2 fusion and shines a light on mesenchymal chondrosarcoma tumorigenesis.
Qi W, Rosikiewicz W, Yin Z, Xu B, Jiang H, Wan S, Fan Y, Wu G, Wang L. Qi W, et al. J Pathol. 2022 Aug;257(5):579-592. doi: 10.1002/path.5899. Epub 2022 Apr 26. J Pathol. 2022. PMID: 35342947 Free PMC article. - Are meningeal hemangiopericytoma and mesenchymal chondrosarcoma the same?: a study of HEY1-NCOA2 fusion.
Fritchie KJ, Jin L, Ruano A, Oliveira AM, Rubin BP. Fritchie KJ, et al. Am J Clin Pathol. 2013 Nov;140(5):670-4. doi: 10.1309/AJCPGUNGP52ZSDNS. Am J Clin Pathol. 2013. PMID: 24124145 - Chromosome aberrations and HEY1-NCOA2 fusion gene in a mesenchymal chondrosarcoma.
Panagopoulos I, Gorunova L, Bjerkehagen B, Boye K, Heim S. Panagopoulos I, et al. Oncol Rep. 2014 Jul;32(1):40-4. doi: 10.3892/or.2014.3180. Epub 2014 May 15. Oncol Rep. 2014. PMID: 24839999 Free PMC article. - Integrating Morphology and Genetics in the Diagnosis of Cartilage Tumors.
de Andrea CE, San-Julian M, Bovée JVMG. de Andrea CE, et al. Surg Pathol Clin. 2017 Sep;10(3):537-552. doi: 10.1016/j.path.2017.04.005. Surg Pathol Clin. 2017. PMID: 28797501 Review. - Mesenchymal Chondrosarcoma: a Review with Emphasis on its Fusion-Driven Biology.
El Beaino M, Roszik J, Livingston JA, Wang WL, Lazar AJ, Amini B, Subbiah V, Lewis V, Conley AP. El Beaino M, et al. Curr Oncol Rep. 2018 Mar 26;20(5):37. doi: 10.1007/s11912-018-0668-z. Curr Oncol Rep. 2018. PMID: 29582189 Review.
Cited by
- Updates on the cytogenetics and molecular cytogenetics of benign and intermediate soft tissue tumors.
Nishio J. Nishio J. Oncol Lett. 2013 Jan;5(1):12-18. doi: 10.3892/ol.2012.1002. Epub 2012 Oct 30. Oncol Lett. 2013. PMID: 23255885 Free PMC article. - Identification of cancer gene fusions based on advanced analysis of the human genome or transcriptome.
Wang L. Wang L. Front Med. 2013 Sep;7(3):280-9. doi: 10.1007/s11684-013-0265-3. Epub 2013 Jun 26. Front Med. 2013. PMID: 23807217 Review. - MAP-Kinase-Driven Hematopoietic Neoplasms: A Decade of Progress in the Molecular Age.
Chakraborty R, Abdel-Wahab O, Durham BH. Chakraborty R, et al. Cold Spring Harb Perspect Med. 2021 May 3;11(5):a034892. doi: 10.1101/cshperspect.a034892. Cold Spring Harb Perspect Med. 2021. PMID: 32601132 Free PMC article. Review. - Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions.
Suehara Y, Arcila M, Wang L, Hasanovic A, Ang D, Ito T, Kimura Y, Drilon A, Guha U, Rusch V, Kris MG, Zakowski MF, Rizvi N, Khanin R, Ladanyi M. Suehara Y, et al. Clin Cancer Res. 2012 Dec 15;18(24):6599-608. doi: 10.1158/1078-0432.CCR-12-0838. Epub 2012 Oct 10. Clin Cancer Res. 2012. PMID: 23052255 Free PMC article. - Case report: A mesenchymal chondrosarcoma with alternative HEY1::NCOA2 fusions in the sella turcica.
Kishikawa S, Kondo A, Yao T, Saito T. Kishikawa S, et al. Pathol Oncol Res. 2024 Aug 6;30:1611730. doi: 10.3389/pore.2024.1611730. eCollection 2024. Pathol Oncol Res. 2024. PMID: 39165647 Free PMC article.
References
- Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, Pollock R, O’Donnell P, Grigoriadis A, Diss T, Eskandarpour M, Presneau N, Hogendoorn PCW, Futreal A, Tirabosco R, Flanagan AM. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011;224:334–343. - PubMed
- Aziz SR, Miremadi AR, McCabe JC. Mesenchymal chondrosarcoma of the maxilla with diffuse metastasis: case report and literature review. J Oral Maxillofac Surg. 2002;60:931–935. - PubMed
- Carapeti M, Aguiar RC, Goldman JM, Cross NC. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood. 1998;91:3127–3133. - PubMed
- Chidambaram A, Sanville P. Mesenchymal chondrosarcoma of the maxilla. J Laryngol Otol. 2000;114:536–539. - PubMed
- Deguchi K, Ayton PM, Carapeti M, Kutok JL, Snyder CS, Williams IR, Cross NC, Glass CK, Cleary ML, Gilliland DG. MOZ-TIF2-induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2-mediated recruitment of CBP. Cancer Cell. 2003;3:259–271. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous