Mutations in POLR3A and POLR3B encoding RNA Polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy - PubMed (original) (raw)
. 2011 Nov 11;89(5):644-51.
doi: 10.1016/j.ajhg.2011.10.003. Epub 2011 Oct 27.
Hitoshi Osaka, Masayuki Sasaki, Jun-Ichi Takanashi, Keisuke Hamada, Akio Yamashita, Hidehiro Shibayama, Masaaki Shiina, Yukiko Kondo, Kiyomi Nishiyama, Yoshinori Tsurusaki, Noriko Miyake, Hiroshi Doi, Kazuhiro Ogata, Ken Inoue, Naomichi Matsumoto
Affiliations
- PMID: 22036171
- PMCID: PMC3213392
- DOI: 10.1016/j.ajhg.2011.10.003
Mutations in POLR3A and POLR3B encoding RNA Polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy
Hirotomo Saitsu et al. Am J Hum Genet. 2011.
Abstract
Congenital hypomyelinating disorders are a heterogeneous group of inherited leukoencephalopathies characterized by abnormal myelin formation. We have recently reported a hypomyelinating syndrome characterized by diffuse cerebral hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum (HCAHC). We performed whole-exome sequencing of three unrelated individuals with HCAHC and identified compound heterozygous mutations in POLR3B in two individuals. The mutations include a nonsense mutation, a splice-site mutation, and two missense mutations at evolutionally conserved amino acids. Using reverse transcription-PCR and sequencing, we demonstrated that the splice-site mutation caused deletion of exon 18 from POLR3B mRNA and that the transcript harboring the nonsense mutation underwent nonsense-mediated mRNA decay. We also identified compound heterozygous missense mutations in POLR3A in the remaining individual. POLR3A and POLR3B encode the largest and second largest subunits of RNA Polymerase III (Pol III), RPC1 and RPC2, respectively. RPC1 and RPC2 together form the active center of the polymerase and contribute to the catalytic activity of the polymerase. Pol III is involved in the transcription of small noncoding RNAs, such as 5S ribosomal RNA and all transfer RNAs (tRNA). We hypothesize that perturbation of Pol III target transcription, especially of tRNAs, could be a common pathological mechanism underlying POLR3A and POLR3B mutations.
Copyright © 2011 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
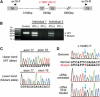
Mutations in POLR3B and POLR3A (A) Pedigrees of four kindreds with HCAHC are shown. We identified four mutations in POLR3B encoding RPC2 in three individuals from two unrelated families and two mutations in POLR3A encoding RPC1 in one family. The segregation of each mutation is shown. (B) Schematic representation of RPC2 (upper) and RPC1 (lower) proteins with Pfam domains (from Ensembl). Locations of each amino-acid-altering mutation are depicted with electropherograms. All of the missense mutations occurred at evolutionally conserved amino acids. Homologous sequences were aligned with the CLUSTALW website. (C–F) 3D representations of RPC1 and RPC2 mutations. Mutated amino acids in RPC1 and RPC2 are shown along with their equivalent positions in the homologous RPB1 and RPB2 subunits of RNA Polymerase II (amino acid and its position in parenthesis). The structure and positions of mutations are illustrated by PyMOL with the crystal structure (PDB accession number 3GTP). RPB3, RPB9, and RPB11 subunits, which are specific to RNA Polymerase II, have been omitted from the figure. RPB1 is shown in green, RPB2 in sky blue, RPB5 in yellow, RPB6 in dark blue, RPB8 in pink, RPB10 in orange, RPB12 in purple, DNA in brown, and RNA in red. Amino acids that interact with mutated amino acids are also shown.
Figure 2
Effects of Splice-Site and Nonsense Mutations in POLR3B (A) Schematic representation of the genomic structure of POLR3B from exon 16 to 19. Exons, introns, and primers are shown by boxes, dashed lines, and arrows, respectively. The mutation in intron 17 is depicted as a red dot. (B) RT-PCR analysis of individuals 1 and 2 with c.1857-2A>C and a normal control. Two PCR products were detected from the individual's cDNA: the upper band is the wild-type (WT) transcript, and the lower band is the mutant. Only a single wild-type amplicon was detected in the control. (C) Sequence of WT and mutant amplicons clearly showed exon 18 skipping in the mutant allele. (D) Analysis of the c.1648C>T mutation. Sequence of PCR products amplified with genomic (upper), cDNA from untreated cells (middle), and cDNA from CHX treated cells (lower) as a template. Although untreated cells show extremely low levels of c.1648C>T mutant allele expression, cells treated to inhibit NMD show significantly increased levels of mutant allele expression.
Figure 3
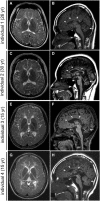
Brain MRI of Individuals with POLR3B and POLR3A Mutations (A, C, E, and G) T2-weighted axial images through the basal ganglia. High-intensity areas in the white matter were observed in all individuals. (B, D, F, and H) T1-weighted midline sagittal images. All the individuals showed hypoplastic corpus callosum (arrowheads) and atrophy of cerebellum (arrows).
Similar articles
- Recessive mutations in POLR3B, encoding the second largest subunit of Pol III, cause a rare hypomyelinating leukodystrophy.
Tétreault M, Choquet K, Orcesi S, Tonduti D, Balottin U, Teichmann M, Fribourg S, Schiffmann R, Brais B, Vanderver A, Bernard G. Tétreault M, et al. Am J Hum Genet. 2011 Nov 11;89(5):652-5. doi: 10.1016/j.ajhg.2011.10.006. Epub 2011 Oct 27. Am J Hum Genet. 2011. PMID: 22036172 Free PMC article. - Mutations of POLR3A encoding a catalytic subunit of RNA polymerase Pol III cause a recessive hypomyelinating leukodystrophy.
Bernard G, Chouery E, Putorti ML, Tétreault M, Takanohashi A, Carosso G, Clément I, Boespflug-Tanguy O, Rodriguez D, Delague V, Abou Ghoch J, Jalkh N, Dorboz I, Fribourg S, Teichmann M, Megarbane A, Schiffmann R, Vanderver A, Brais B. Bernard G, et al. Am J Hum Genet. 2011 Sep 9;89(3):415-23. doi: 10.1016/j.ajhg.2011.07.014. Am J Hum Genet. 2011. PMID: 21855841 Free PMC article. - Mutations in POLR3A and POLR3B are a major cause of hypomyelinating leukodystrophies with or without dental abnormalities and/or hypogonadotropic hypogonadism.
Daoud H, Tétreault M, Gibson W, Guerrero K, Cohen A, Gburek-Augustat J, Synofzik M, Brais B, Stevens CA, Sanchez-Carpintero R, Goizet C, Naidu S, Vanderver A, Bernard G. Daoud H, et al. J Med Genet. 2013 Mar;50(3):194-7. doi: 10.1136/jmedgenet-2012-101357. Epub 2013 Jan 25. J Med Genet. 2013. PMID: 23355746 - POLR3-related leukodystrophy: How do mutations affecting RNA polymerase III subunits cause hypomyelination?
Coulombe B, Derksen A, La Piana R, Brais B, Gauthier MS, Bernard G. Coulombe B, et al. Fac Rev. 2021 Feb 5;10:12. doi: 10.12703/r/10-12. eCollection 2021. Fac Rev. 2021. PMID: 33659930 Free PMC article. Review. - RNA Polymerase III Subunit Mutations in Genetic Diseases.
Lata E, Choquet K, Sagliocco F, Brais B, Bernard G, Teichmann M. Lata E, et al. Front Mol Biosci. 2021 Jul 30;8:696438. doi: 10.3389/fmolb.2021.696438. eCollection 2021. Front Mol Biosci. 2021. PMID: 34395528 Free PMC article. Review.
Cited by
- Tremor Ataxia With Central Hypomyelation Phenotype Related to a Recurrent POLR3A Mutation in Six Unrelated Tunisian Families.
Kraoua I, Jamoussi M, Drissi C, Kraoua L, Drunat S, Benrhouma H, Ben Younes T, Nagi S, Abdelhak S, Boespflug Tanguy O, Youssef-Turki IB, Trabelsi M, Dorboz I. Kraoua I, et al. Mol Genet Genomic Med. 2024 Oct;12(10):e70007. doi: 10.1002/mgg3.70007. Mol Genet Genomic Med. 2024. PMID: 39436788 Free PMC article. - tRNA dysregulation and disease.
Orellana EA, Siegal E, Gregory RI. Orellana EA, et al. Nat Rev Genet. 2022 Nov;23(11):651-664. doi: 10.1038/s41576-022-00501-9. Epub 2022 Jun 9. Nat Rev Genet. 2022. PMID: 35681060 Free PMC article. Review. - Transcription termination by the eukaryotic RNA polymerase III.
Arimbasseri AG, Rijal K, Maraia RJ. Arimbasseri AG, et al. Biochim Biophys Acta. 2013 Mar-Apr;1829(3-4):318-30. doi: 10.1016/j.bbagrm.2012.10.006. Epub 2012 Oct 23. Biochim Biophys Acta. 2013. PMID: 23099421 Free PMC article. Review. - Longitudinal follow up of a boy affected by Pol III-related leukodystrophy: a detailed phenotype description.
Battini R, Bertelloni S, Astrea G, Casarano M, Travaglini L, Baroncelli G, Pasquariello R, Bertini E, Cioni G. Battini R, et al. BMC Med Genet. 2015 Jul 25;16:53. doi: 10.1186/s12881-015-0203-0. BMC Med Genet. 2015. PMID: 26204956 Free PMC article. - Cockayne syndrome group A and B proteins converge on transcription-linked resolution of non-B DNA.
Scheibye-Knudsen M, Tseng A, Borch Jensen M, Scheibye-Alsing K, Fang EF, Iyama T, Bharti SK, Marosi K, Froetscher L, Kassahun H, Eckley DM, Maul RW, Bastian P, De S, Ghosh S, Nilsen H, Goldberg IG, Mattson MP, Wilson DM 3rd, Brosh RM Jr, Gorospe M, Bohr VA. Scheibye-Knudsen M, et al. Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12502-12507. doi: 10.1073/pnas.1610198113. Epub 2016 Oct 18. Proc Natl Acad Sci U S A. 2016. PMID: 27791127 Free PMC article.
References
- Wolf N.I., Harting I., Boltshauser E., Wiegand G., Koch M.J., Schmitt-Mechelke T., Martin E., Zschocke J., Uhlenberg B., Hoffmann G.F., et al. Leukoencephalopathy with ataxia, hypodontia, and hypomyelination. Neurology. 2005;64:1461–1464. - PubMed
- Wolf N.I., Harting I., Innes A.M., Patzer S., Zeitler P., Schneider A., Wolff A., Baier K., Zschocke J., Ebinger F., et al. Ataxia, delayed dentition and hypomyelination: a novel leukoencephalopathy. Neuropediatrics. 2007;38:64–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases