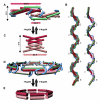
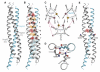Molecular architecture of the transport channel of the nuclear pore complex - PubMed (original) (raw)
Molecular architecture of the transport channel of the nuclear pore complex
Sozanne R Solmaz et al. Cell. 2011.
Abstract
The nuclear pore complex encloses a central channel for nucleocytoplasmic transport, which is thought to consist of three nucleoporins, Nup54, Nup58, and Nup62. However, the structure and composition of the channel are elusive. We determined the crystal structures of the interacting domains between these nucleoporins and pieced together the molecular architecture of the mammalian transport channel. Located in the channel midplane is a flexible Nup54⋅Nup58 ring that can undergo large rearrangements yielding diameter changes from ∼20 to ∼40 nm. Nup62⋅Nup54 triple helices project alternately up and down from either side of the midplane ring and form nucleoplasmic and cytoplasmic entries. The channel consists of as many as 224 copies of the three nucleoporins, amounting to a molar mass of 12.3 MDa and contributing 256 phenylalanine-glycine repeat regions. We propose that the occupancy of these repeat regions with transport receptors modulates ring diameter and transport activity.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
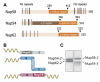
Figure 1. Mapping of Interacting Domains
(A) Domain representation of Nup58, Nup54, and Nup62 from Rattus norvegicus indicating α-helical (dark orange), α/β (brown), and unstructured regions (light orange) with FG repeats (black lines) (Melčák et al., 2007). (B) Schematic representation of binding sites on Nup54 (blue) for Nup62 (gray) and Nup58 (pink). The α-helical and the unstructured regions with FG repeats are visualized as rods and coils, respectively. (C) Coexpressed and purified Nup62-_2_·Nup54-2 (left) and Nup54-_3_·Nup58-2 (right) complexes were analyzed by SDS-PAGE and stained by Coomassie blue. See also Figure S1.
Figure 2. Structure of Nup62-2·Nup54-2
(A) Ribbon representation of the asymmetric unit consisting of one Nup54-2 α helix (cyan) and two Nup62-2 α helices (gray). The N termini are labeled. (B) Buried residues of the heterotrimer are shown as van der Waals spheres. Residues of the coiled-coil interface at a and d position of heptad repeat are colored in red and orange, respectively. Layers of the coiled-coil region are labeled from 1 to 7. Layer 5 is labeled as “Q” and is highlighted by a dashed box. (C) Helical wheel representation of the coiled-coil region of the Nup62-_2_·Nup54-2 complex, viewed from the N terminus. The side chains of residues at a and d positions are segregated into nonequivalent layers, forming a predominantly hydrophobic core. Charged residues on the surface of the coiled coil that are involved in ionic interactions between adjacent α helices (red dashed lines) are marked as red circles. (D) Cross-section of the Q layer showing hydrogen bond network. Side chains of the Gln residues and two main chain oxygen atoms of the Q layer are shown in stick-and-ball representation; backbone is shown as a main chain Ca trace colored as in (A). Hydrogen bonds are shown as red dashed lines, and bond lengths are indicated. (E) Ribbon representation of the Nup62-_2_·Nup54-2 structure colored as in (A), showing the position of Q394. Residues Q394 of two Nup62 molecules are shown in stick-and-ball representation, and the residues of the Q layer are shown as red van der Waals spheres. See also Figures S2 and S3 and Tables S1, S2, and S3.
Figure 3. Structure of Nup54-3·Nup58-2
(A) The asymmetric unit consists of one Nup58-2 (red) protomer and two Nup54-3 protomers of different conformation (straight Nup54-3, green; bent Nup54-3, cyan). A symmetry-related unit is shown in gray and 2-fold axis of symmetry in black. (B) Cartoon representation of the crystallographic hexamer, in which a heterotetramer consisting of two straight Nup54-3 conformers (green) and two Nup58-2 hairpins (red) forms a compact substructure. Nup54-3 caps are shown in gray. (C) Structure of the crystallographic dodecamer. Three 2-fold axes of symmetry are indicated in black. Due to 2-fold symmetry, the dodecamer defines all interhexameric contacts. (D) Residues that mediate hydrophobic (blue) and electrostatic (orange) interactions in the Nup54-_3_·Nup58-2 heterotetramer are shown in van der Waals sphere representation. The backbones of the heterotetramer (Nup58-2, red; straight Nup54-3, green) and bent Nup54-3 caps (gray) are indicated. (E) Nature of the interactions between two Nup58-_2_·Nup54-3 heterotetramers and the two Nup54-3 clamps within the dodecamer. Interacting residues are indicated as in (D). See also Figure S4 and Table S1.
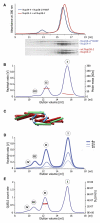
Figure 4. Analysis of Higher-Order Assemblies in Solution
(A) Size exclusion chromatography (top) of WT Nup58-2 (red) or Nup58-2/ Y406F (blue), each mixed with Nup54-4, in 1:2 molar ratio (2 mg/ml each); SDS-PAGE analyses of fractions are shown in the bottom panels. (B) Nup58-2/Y406F and Nup54-4 were mixed (2 mg/ml each) and analyzed by size exclusion chromatography coupled to multiangle light scattering, yielding three peaks (I, II, and III). The Rayleigh ratio (blue) and the molar mass (red) versus the elution volume for one experiment are shown. (C) Cartoon representation of the decamer. (D) Concentration-dependent higher-order Nup54·Nup58 oligomerization. Nup58-2/Y406F and Nup54-4 were mixed at increasing concentrations (1x corresponds to 2 mg/ml each) and analyzed as in (B). (E) Nup58-2/Y406F and Nup54-4 were mixed (at 8 mg/ml each) and analyzed by size exclusion chromatography coupled to dynamic light scattering. The dynamic light scattering (QUELS) count rate (blue) and translational diffusion coefficient DT (red) versus the elution volume for one experiment are shown. See also Figures S5 and S6 and Table S2.
Figure 5. Conformational Changes of Nup58 Induced by Binding to Nup54
(A) Structure of the Nup58-2 dimer (Melčák et al., 2007). (B) In the Nup58-_2_·Nup54-3 heterotetramer, each Nup58-2 molecule (red) folds into a coiled coil. a position residues (orange) and d position residues (gray) are shown as van der Waals spheres. Nup54-3 molecules are shown in green. Boxed regions in (A) and (B) are expanded in (C) and (D), respectively. (C) Tyr346 residues (blue) of two Nup58-2 molecules (red and pink α helices) form a hydrogen bond (dashed line) within a nonpolar interface of the Nup58 homodimer, indicated by gray residues. (D) In the Nup54-_3_·Nup58-2 complex, an alternative polar partner for Tyr346 (blue) is provided by residue Gln473 (blue) of Nup54 (green α helix). Nonpolar interface is indicated by gray residues. (E) Structure of Nup58-2 dimer (oriented as in A), in which one protomer is colored in a gradient according to the main-chain B factors. Blue and red represent the lowest (14Å2) and highest (74Å2) B factor values, respectively. (F) Nup58-_2_·Nup54-3 heterotetramer (oriented as in B), in which one Nup58-2 and one Nup54-3 protomer are colored according to the main chain B factors ranging from 32Å (blue) to 115Å2 (red). (G) Bent Nup54-3 colored according to the main-chain B factors as in (F). See also Figure S7.
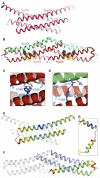
Figure 6. Alternative Interactions of Nup58 and Nup54 Can Give Rise to Large Diameter Changes within the NPC
(A) Cartoon representation of a Nup54·Nup58 dodecamer, which corresponds to 1/8 segment of the midplane ring. Within the dodecamer, two Nup54·Nup58 heterotetramers (Nup58 molecules of consecutive tetramers in red or pink; straight Nup54 conformers, green) are clamped together by bent Nup54 (cyan). The dimensions of the heterotetramer(s) and their overlap are indicated (measured between main-chain Ca atoms). (B) Surface representation of a higher-order oligomer, derived from the X-ray structure (left) and its schematic representation (right), in which individual Nup54·Nup58 heterotetramers are depicted as single cylinders and the bent Nup54 clamps are depicted as curved tubes, colored according to (A). Eight segments are shown. (C) Cartoon representation of the Nup58 homotetramer (dimers in pink and red) (Melčák et al., 2007). (D) Schematic representation of a Nup54·Nup58 heterooligomer ring. Nup54·Nup58 heterotetramers are depicted as single cylinders (the green part represents two Nup54 protomers, and the red or pink part represents two Nup58 hairpins with alternating color in consecutive heterotetramers). Nup54 clamps are depicted as curved tubes (cyan). (E) Circumferential arrangement of eight Nup58 homotetramers (dimers schematically shown as single cylinders in pink or red). See also Movies S1 and S2.
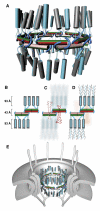
Figure 7. Architecture of the Mammalian Transport Channel Formed by the Nup62·Nup54·Nup58 Complex
(A) Cartoon representation of the transport channel with color coding and representation of the midplane ring as in Figure 6D. Nup62·Nup54 heterotrimers are shown as vertical cylinders colored in gray (two Nup62 molecules) and cyan (one Nup54). The linker regions of the Nup54 molecules are represented as cyan and green transparent tubes. See also Movie S3. (B) Schematic representation of 1/8 segment of the transport channel. Nup54·Nup58 heterotetramers are shown as boxes in same color coding as in (A). Nup54 clamps are shown as cyan hooks. The Nup62·Nup54 heterotrimer is represented by three cylinders (Nup62 and Nup54 in gray and cyan, respectively). Dashed lines represent linker regions. (C) Schematic arrangements of FG repeat regions of Nup58 in the transport channel. Both N-terminal (longer ribbons) and C-terminal (shorter ribbons) FG regions are colored pink or red. Dashed pink and red lines indicate N-terminal α-helical regions of Nup58 that connect Nup58 to N-terminal FG regions. Nup62 and part of Nup54 molecules are depicted in background. (D) The position of FG repeat regions of Nup54 and Nup62. The N-terminal FG regions (ribbons) of Nup54 (cyan) and Nup62 (gray) project to cytoplasmic and nucleoplasmic sides of the midplane ring. FG regions of Nup58 are shown in the background (pink). (E) The transport channel (as shown in A) embedded in the NPC. The core of the NPC is schematically shown as concentric cylinders and is anchored in the nuclear envelope. Attached to the core are a cytoplasmic ring with filaments and a nucleoplasmic ring with the “nuclear basket.”
Similar articles
- Ordered Regions of Channel Nucleoporins Nup62, Nup54, and Nup58 Form Dynamic Complexes in Solution.
Sharma A, Solmaz SR, Blobel G, Melčák I. Sharma A, et al. J Biol Chem. 2015 Jul 24;290(30):18370-8. doi: 10.1074/jbc.M115.663500. Epub 2015 May 29. J Biol Chem. 2015. PMID: 26025361 Free PMC article. - Ring cycle for dilating and constricting the nuclear pore.
Solmaz SR, Blobel G, Melcák I. Solmaz SR, et al. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):5858-63. doi: 10.1073/pnas.1302655110. Epub 2013 Mar 11. Proc Natl Acad Sci U S A. 2013. PMID: 23479651 Free PMC article. - The Nup62 Coiled-Coil Motif Provides Plasticity for Triple-Helix Bundle Formation.
Dewangan PS, Sonawane PJ, Chouksey AR, Chauhan R. Dewangan PS, et al. Biochemistry. 2017 Jun 6;56(22):2803-2811. doi: 10.1021/acs.biochem.6b01050. Epub 2017 Apr 26. Biochemistry. 2017. PMID: 28406021 - The Part and the Whole: functions of nucleoporins in nucleocytoplasmic transport.
Wälde S, Kehlenbach RH. Wälde S, et al. Trends Cell Biol. 2010 Aug;20(8):461-9. doi: 10.1016/j.tcb.2010.05.001. Epub 2010 Jun 4. Trends Cell Biol. 2010. PMID: 20627572 Review. - The Structure of the Nuclear Pore Complex (An Update).
Lin DH, Hoelz A. Lin DH, et al. Annu Rev Biochem. 2019 Jun 20;88:725-783. doi: 10.1146/annurev-biochem-062917-011901. Epub 2019 Mar 18. Annu Rev Biochem. 2019. PMID: 30883195 Free PMC article. Review.
Cited by
- HTLV-1 Rex: the courier of viral messages making use of the host vehicle.
Nakano K, Watanabe T. Nakano K, et al. Front Microbiol. 2012 Sep 6;3:330. doi: 10.3389/fmicb.2012.00330. eCollection 2012. Front Microbiol. 2012. PMID: 22973269 Free PMC article. - Gold nanoparticles to improve HIV drug delivery.
Garrido C, Simpson CA, Dahl NP, Bresee J, Whitehead DC, Lindsey EA, Harris TL, Smith CA, Carter CJ, Feldheim DL, Melander C, Margolis DM. Garrido C, et al. Future Med Chem. 2015;7(9):1097-107. doi: 10.4155/fmc.15.57. Future Med Chem. 2015. PMID: 26132521 Free PMC article. - Mutated NUP188 and Other Nucleoporins as Gateways to Developmental Syndromes.
Poot M. Poot M. Mol Syndromol. 2020 Feb;11(1):1-3. doi: 10.1159/000506410. Epub 2020 Feb 15. Mol Syndromol. 2020. PMID: 32256295 Free PMC article. No abstract available. - ROCK-dependent phosphorylation of NUP62 regulates p63 nuclear transport and squamous cell carcinoma proliferation.
Hazawa M, Lin DC, Kobayashi A, Jiang YY, Xu L, Dewi FRP, Mohamed MS, Hartono, Nakada M, Meguro-Horike M, Horike SI, Koeffler HP, Wong RW. Hazawa M, et al. EMBO Rep. 2018 Jan;19(1):73-88. doi: 10.15252/embr.201744523. Epub 2017 Dec 7. EMBO Rep. 2018. PMID: 29217659 Free PMC article. - Traumatic injury compromises nucleocytoplasmic transport and leads to TDP-43 pathology.
Anderson EN, Morera AA, Kour S, Cherry JD, Ramesh N, Gleixner A, Schwartz JC, Ebmeier C, Old W, Donnelly CJ, Cheng JP, Kline AE, Kofler J, Stein TD, Pandey UB. Anderson EN, et al. Elife. 2021 May 26;10:e67587. doi: 10.7554/eLife.67587. Elife. 2021. PMID: 34060470 Free PMC article.
References
- Akey DL, Malashkevich VN, Kim PS. Buried polar residues in coiled-coil interfaces. Biochemistry. 2001;40:6352–6360. - PubMed
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007;450:695–701. - PubMed
- Basel-Vanagaite L, Muncher L, Straussberg R, Pasmanik-Chor M, Yahav M, Rainshtein L, Walsh CA, Magal N, Taub E, Drasinover V, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann. Neurol. 2006;60:214–222. - PubMed
- Bayliss R, Littlewood T, Stewart M. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 2000;102:99–108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases