Nitroglycerin drives endothelial nitric oxide synthase activation via the phosphatidylinositol 3-kinase/protein kinase B pathway - PubMed (original) (raw)
. 2012 Jan 15;52(2):427-35.
doi: 10.1016/j.freeradbiomed.2011.09.020. Epub 2011 Oct 7.
Varadarajan Sudhahar, Kristine Ansenberger-Fricano, Denise C Fernandes, Leonardo Y Tanaka, Tohru Fukai, Francisco R M Laurindo, Ronald P Mason, Jeannette Vasquez-Vivar, Richard D Minshall, Krisztian Stadler, Marcelo G Bonini
Affiliations
- PMID: 22037515
- PMCID: PMC3432314
- DOI: 10.1016/j.freeradbiomed.2011.09.020
Nitroglycerin drives endothelial nitric oxide synthase activation via the phosphatidylinositol 3-kinase/protein kinase B pathway
Mao Mao et al. Free Radic Biol Med. 2012.
Abstract
Nitroglycerin (GTN) has been clinically used to treat angina pectoris and acute heart episodes for over 100 years. The effects of GTN have long been recognized and active research has contributed to the unraveling of numerous metabolic routes capable of converting GTN to the potent vasoactive messenger nitric oxide. Recently, the mechanism by which minute doses of GTN elicit robust pharmacological responses was revisited and eNOS activation was implicated as an important route mediating vasodilation induced by low GTN doses (1-50nM). Here, we demonstrate that at such concentrations the pharmacologic effects of nitroglycerin are largely dependent on the phosphatidylinositol 3-kinase, Akt/PKB, and phosphatase and tensin homolog deleted on chromosome 10 (PTEN) signal transduction axis. Furthermore, we demonstrate that nitroglycerin-dependent accumulation of 3,4,5-InsP(3), probably because of inhibition of PTEN, is important for eNOS activation, conferring a mechanistic basis for GTN pharmacological action at pharmacologically relevant doses.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Fig. 1
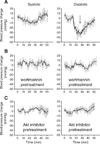
Intracellular NO measurement by HPLC analysis of DAF-2 T (triazolofluorescein). HAEC were pretreated with wortmannin (500 nM), Akt inhibitor (20 µM), or L-NIO (0.1 mM) before addition of DAF-2 (5 µM) and tandem treatments with GTN (10 nM) or VEGF (20 ng/ml). Cellular concentrations of the product DAF-2 T were analyzed by HPLC and calculated using a standard curve. Reported data are DAF-2 T concentration normalized to protein content in each sample. *P<0.05, **P<0.01.
Fig. 2
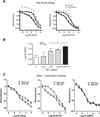
PI3K involvement in GTN-dependent vasodilation. (A) Effect of wortmannin (PI3K inhibitor) pretreatment upon the acetylcholine (Ach)- or GTN-induced dilation of rat aortic rings. (B) Nitroglycerin EC50 values measured in rat aortic rings in the presence of the indicated concentrations of Akt 1/2 inhibitor. (C) Vasodilation experiment with mesenteric tissue recovered from p110γ-knockout mice. p110γ is the catalytic subunit of endothelial PI3K enzyme. Both pharmacologic inhibition and genetic knockout of PI3K inhibited GTN-induced dilation of conducing (aorta) and resistant (mesenteric artery) vessels. *P<0.05, **P<0.01.
Fig. 3
Assessment of PI3K and Akt inhibition effects upon GTN-induced blood pressure decreases in anesthetized rats. Experiments were performed using the tail-cuff method. Animals were anesthetized with ketamine/xylazine mixtures according to the Materials and methods. Blood pressure was stabilized for at least 20 min before GTN administration. Wortmannin (0.5 mg/kg) or Akt inhibitor (2 mg/kg) was administrated 2 h before the experiments in DMSO (50 µL injection volume). Controls received DMSO injection equivalent to the amount required for inhibitor treatment 2 h before GTN.
Fig. 4
PI3K/Akt-dependent phosphorylation of eNOS elicited by nitroglycerin. BAEC were challenged with nitroglycerin (500 nM) for 3 min before harvest. PI3K inhibitor wortmannin (100 nM) or Akt inhibitor (20 µM) was dissolved in DMSO and added to the cell cultures 1 h before GTN treatment; DMSO was also added at the same concentration (v/v) to control groups. Final DMSO concentration did not exceed 0.1%.
Fig. 5
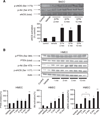
Concentration-dependent Akt activation by GTN. (A) BAEC cells were treated with vehicle control (30% ethanol, 30% propylene glycol, 40% water) or the indicated GTN concentrations for 3 min before harvest. (B) Band densities were measured using ImageJ software. Results were quantified as relative Akt activation compared with control; *P<0.05 by Student's t test.
Fig. 6
Time-dependent activation of Akt and eNOS paralleling PTEN phosphorylation by GTN. Representative Western blots showing PTEN phosphorylation (Ser 380), eNOS phosphorylation (Ser 1177 or 1179), and Akt phosphorylation (Ser 473) in (A) BAEC and (B) HMEC treated with vehicle or 500 nM GTN for the indicated amounts of time. Vehicle was added for 10 min in (A) and 15 min in (B). Results show rapid and sustained eNOS phosphorylation at the activation site Ser 1177, Akt phosphorylation at the activation site Ser 473, and PTEN phosphorylation at the inhibitory site Ser 380 by 500 nM GTN. Band intensities for phosphorylated eNOS in BAEC and for the experiments performed with HMEC were quantified using ImageJ and the values are reported as density units relative to control; *P<0.05, **P<0.01.
Fig. 7
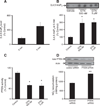
GTN treatment elaborates cellular 3,4,5-InsP3 level by inhibiting PTEN activity. (A) Measurement of 3,4,5-InsP3 level as a time-dependent response to GTN treatment. HMEC were treated with 500 nM GTN for 2 and 5 min. Results are shown relative to control. (B) Mass strip blot of PTEN substrate 3,4,5-InsP3 in HMEC treated with vehicle or GTN for 15 min. Results are shown relative to control. (C) Measurement of immunoprecipitated PTEN phosphatase activity by Biomol green assay. Three independent experiments were performed and the results reveal PTEN inhibition consistent with increase in 3,4,5-InsP3 levels. The values are reported as phosphate release relative to control; *P<0.05 by Student's t test. (D) PTEN silencing by siRNA in MEC and subsequent measurement of basal NO production was assessed by the chemiluminescence-based quantification of NO2− accumulation in medium. Results show mean values of four independent measurements; **P<0.01. Quantification of band densities was performed using Image software.
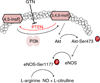
Fig. 8
Representation of the proposed mechanism of GTN-induced eNOS activation via PTEN inhibition.
Similar articles
- Depolarization of mitochondria in endothelial cells promotes cerebral artery vasodilation by activation of nitric oxide synthase.
Katakam PV, Wappler EA, Katz PS, Rutkai I, Institoris A, Domoki F, Gáspár T, Grovenburg SM, Snipes JA, Busija DW. Katakam PV, et al. Arterioscler Thromb Vasc Biol. 2013 Apr;33(4):752-9. doi: 10.1161/ATVBAHA.112.300560. Epub 2013 Jan 17. Arterioscler Thromb Vasc Biol. 2013. PMID: 23329133 Free PMC article. - Astragaloside IV Improves Vasodilatation Function by Regulating the PI3K/Akt/eNOS Signaling Pathway in Rat Aorta Endothelial Cells.
Lin XP, Cui HJ, Yang AL, Luo JK, Tang T. Lin XP, et al. J Vasc Res. 2018;55(3):169-176. doi: 10.1159/000489958. Epub 2018 Jul 4. J Vasc Res. 2018. PMID: 29972829 - Rumex acetosa L. induces vasorelaxation in rat aorta via activation of PI3-kinase/Akt- AND Ca(2+)-eNOS-NO signaling in endothelial cells.
Sun YY, Su XH, Jin JY, Zhou ZQ, Sun SS, Wen JF, Kang DG, Lee HS, Cho KW, Jin SN. Sun YY, et al. J Physiol Pharmacol. 2015 Dec;66(6):907-15. J Physiol Pharmacol. 2015. PMID: 26769840 - CCN1 acutely increases nitric oxide production via integrin αvβ3-Akt-S6K-phosphorylation of endothelial nitric oxide synthase at the serine 1177 signaling axis.
Hwang S, Lee HJ, Kim G, Won KJ, Park YS, Jo I. Hwang S, et al. Free Radic Biol Med. 2015 Dec;89:229-40. doi: 10.1016/j.freeradbiomed.2015.08.005. Epub 2015 Sep 21. Free Radic Biol Med. 2015. PMID: 26393424 - Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles.
LeBlanc AJ, Shipley RD, Kang LS, Muller-Delp JM. LeBlanc AJ, et al. Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2280-8. doi: 10.1152/ajpheart.00541.2008. Epub 2008 Oct 3. Am J Physiol Heart Circ Physiol. 2008. PMID: 18835919 Free PMC article.
Cited by
- The role of vascular endothelium in nitroglycerin-mediated vasodilation.
Zhou K, Parker JD. Zhou K, et al. Br J Clin Pharmacol. 2019 Feb;85(2):377-384. doi: 10.1111/bcp.13804. Epub 2018 Dec 3. Br J Clin Pharmacol. 2019. PMID: 30378151 Free PMC article. Clinical Trial. - Nitroglycerin tolerance in caveolin-1 deficient mice.
Mao M, Varadarajan S, Fukai T, Bakhshi FR, Chernaya O, Dudley SC Jr, Minshall RD, Bonini MG. Mao M, et al. PLoS One. 2014 Aug 26;9(8):e104101. doi: 10.1371/journal.pone.0104101. eCollection 2014. PLoS One. 2014. PMID: 25158065 Free PMC article. - Transforming growth factor-β regulates endothelial function during high salt intake in rats.
Ying WZ, Aaron KJ, Sanders PW. Ying WZ, et al. Hypertension. 2013 Nov;62(5):951-6. doi: 10.1161/HYPERTENSIONAHA.113.01835. Epub 2013 Sep 16. Hypertension. 2013. PMID: 24041947 Free PMC article. - Coronary Serum Exosomes Derived from Patients with Myocardial Ischemia Regulate Angiogenesis through the miR-939-mediated Nitric Oxide Signaling Pathway.
Li H, Liao Y, Gao L, Zhuang T, Huang Z, Zhu H, Ge J. Li H, et al. Theranostics. 2018 Mar 7;8(8):2079-2093. doi: 10.7150/thno.21895. eCollection 2018. Theranostics. 2018. PMID: 29721064 Free PMC article. - Cataract induction by administration of nitroglycerin in cardiac patients through imbalance in redox status.
El-Gharabawy RM, Ahmed AS, Al-Najjar AH. El-Gharabawy RM, et al. Ther Clin Risk Manag. 2016 Sep 27;12:1487-1496. doi: 10.2147/TCRM.S114469. eCollection 2016. Ther Clin Risk Manag. 2016. PMID: 27729797 Free PMC article.
References
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ. Res. 2003;93:e104–e112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous