Ulcerogenic Helicobacter pylori strains isolated from children: a contribution to get insight into the virulence of the bacteria - PubMed (original) (raw)
Ulcerogenic Helicobacter pylori strains isolated from children: a contribution to get insight into the virulence of the bacteria
Inês Vitoriano et al. PLoS One. 2011.
Abstract
Infection with Helicobacter pylori is the major cause for the development of peptic ulcer disease (PUD). In children, with no other etiology for the disease, this rare event occurs shortly after infection. In these young patients, habits of smoking, diet, consumption of alcohol and non-steroid anti-inflammatory drugs and stress, in addition to the genetic susceptibility of the patient, represent a minor influence. Accordingly, the virulence of the implicated H. pylori strain should play a crucial role in the development of PUD. Corroborating this, our in vitro infection assays comparing a pool of five H. pylori strains isolated from children with PUD to a pool of five other pediatric clinical isolates associated with non-ulcer dyspepsia (NUD) showed the greater ability of PUD strains to induce a marked decrease in the viability of gastric cells and to cause severe damage in the cells cytoskeleton as well as an impairment in the production/secretion of mucins. To uncover virulence features, we compared the proteome of these two groups of H. pylori strains. Two-dimensional gel electrophoresis followed by mass-spectrometry allowed us to detect 27 differentially expressed proteins between them. In addition to the presence of genes encoding well established virulence factors, namely cagA, vacAs1, oipA "on" status, homB and jhp562 genes, the pediatric ulcerogenic strains shared a proteome profile characterized by changes in the abundance of: motility-associated proteins, accounting for higher motility; antioxidant proteins, which may confer increased resistance to inflammation; and enzymes involved in key steps in the metabolism of glucose, amino acids and urea, which may be advantageous to face fluctuations of nutrients. In conclusion, the enhanced virulence of the pediatric ulcerogenic H. pylori strains may result from a synergy between their natural ability to better adapt to the hostile human stomach and the expression of the established virulence factors.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
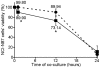
Figure 1. Impact of the two isogenic groups of pediatric H. pylori strains on NCI-N87 cells viability.
The viability of NCI-N87 cells was assessed by the trypan blue exclusion assay after 1, 12 and 24 h of co-culture with a pool of five pediatric PUD-associated strains (▪ in full line) and a pool of five pediatric NUD-associated strains (• in dashed line). For normalization, the value of 100% corresponds to the viability of non-infected NCI-N87 cells. * indicates values that were determined as significantly different (p<0.05) between the viability of cells infected with PUD strains and that of cells infected with NUD-strains at the same time-point of co-culture. Symbols and error bars are means ± SD of the values at each point for 3 observations.
Figure 2. Impact of the two isogenic groups of pediatric H. pylori strains on the NCI-N87 cells morphology.
NCI-N87 cells grown to 80–90% confluence were co-cultured for 24 h with the pool of five isogenic NUD-associated H. pylori strains (NUD) and in parallel with the pool of five isogenic PUD strains (PUD). The control corresponds to non-infected NCI-N87 cells. A), B), and C) light microscopy observations. D), E), and F) immunodetection of the microtubule network (green) (1∶1000 α-tubulin antibody plus a FITC conjugated secondary antibody). G), H), and I) PAS staining alone, and in J), L), and M) in conjugation with hematoxylin. Dark arrow indicates extracellular vesicles that reacted with PAS.
Figure 3. Profile of the soluble proteome of the 10 studied pediatric H. pylori strains.
These include five strains associated with NUD (173/00, 207/99, 228/99, 655/99 and 1786/05), four from patients affected by DU (1089/03, 1152/04, 1198/04 and 1846/05) and a strain associated with GU (499/02). Proteins from the total biomass recovered from a 24 h grown plate were separated by 2DE, i.e., first in a non-linear pH 3–11 and than in a 7–16% (w/v) SDS-PAGE. After CBB-staining, gels were analyzed using ImageMaster™ 2-D Platinum software. Black arrow indicates spots that were more abundant in PUD (DU+GU) strains when compared to NUD strains; Dotted arrow indicates spots that were less abundant in PUD (DU+GU) strains when compared to NUD strains; Dashed arrow indicates the spot that was shifted right in DU strains when compared to NUD strains. Spot numbers indicated in the 2DE maps are the same as used in Table 2.
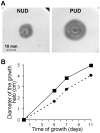
Figure 4. Motility of the two isogenic groups of pediatric H. pylori strains in study.
The five strains of each class, recovered from 24 h grown plates, were pooled together, inoculated on semi-solid BHI broth −5% FBS medium plates and incubated for 11 days at 37°C under microaerophilic conditions. A) The growth halo observed at day 5 for the pool of PUD and of NUD strains. B) Variation of the diameter of the growth halo along the time (▪ in full line refers to PUD strains and • in dashed line refers to NUD strains). Symbols are means of the values at each point for 2 observations.
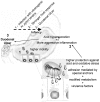
Figure 5. Schematic diagram of the DU development in children upon H. pylori infection.
The virulence of the pediatric PUD-associated H. pylori strains results from a synergy between their natural ability to better adapt to the hostile human stomach and their virulence factors. Adaptation is ensured by: higher motility (↑ FlaA, ↑ FlgE, ↓ HELP_0944, ↑ HPAG1_1081, and ↑ HPG27_1480), higher ability to survive under low pH conditions (↑ UreA and B and ↑ putative aldo-keto reductase), better antioxidant defenses against inflammation (↑ Pfr, ↑ NapA and ↓ KatA), modified metabolism (↓ HyuA, ↓ AspA, ↑ Pgk, ↓ ScoA and B, ↑AroQ, ↓ Porγ, ↑ FldA, ↓ RpsA and ↓ CysS), adhesion (indicated by a anchor) mediated by “on” OipA and HomB. Virulence factors (indicated by a bomb): cagA and VacAs1. 1 – Infection; 2 – Host response (acid hypersecretion and inflammation); 3 – duodenal acid injury (Duodenal Ulcer, indicated by an explosion symbol). Dotted arrow - Time line.
Similar articles
- BabA-mediated adherence of pediatric ulcerogenic H. pylori strains to gastric mucins at neutral and acidic pH.
Quintana-Hayashi MP, Rocha R, Padra M, Thorell A, Jin C, Karlsson NG, Roxo-Rosa M, Oleastro M, Lindén SK. Quintana-Hayashi MP, et al. Virulence. 2018;9(1):1699-1717. doi: 10.1080/21505594.2018.1532243. Virulence. 2018. PMID: 30298790 Free PMC article. - Evaluation of the clinical significance of homB, a novel candidate marker of Helicobacter pylori strains associated with peptic ulcer disease.
Oleastro M, Cordeiro R, Ferrand J, Nunes B, Lehours P, Carvalho-Oliveira I, Mendes AI, Penque D, Monteiro L, Mégraud F, Ménard A. Oleastro M, et al. J Infect Dis. 2008 Nov 1;198(9):1379-87. doi: 10.1086/592166. J Infect Dis. 2008. PMID: 18811585 - Prevalence of Helicobacter pylori cagA, vacA, iceA, babA2 genotypes in Polish children and adolescents with gastroduodenal disease.
Biernat MM, Gościniak G, Iwańczak B. Biernat MM, et al. Postepy Hig Med Dosw (Online). 2014 Aug 22;68:1015-21. doi: 10.5604/17322693.1118211. Postepy Hig Med Dosw (Online). 2014. PMID: 25228509 - Identifiable Helicobacter pylori strains or factors important in the development of duodenal ulcer disease.
Figura N. Figura N. Helicobacter. 1997 Jul;2 Suppl 1:S3-12. doi: 10.1111/j.1523-5378.1997.06b06.x. Helicobacter. 1997. PMID: 9432351 Review. - [Peptic Ulcer Disease Associated with Helicobacter pylori Infection].
Yeo SH, Yang CH. Yeo SH, et al. Korean J Gastroenterol. 2016 Jun 25;67(6):289-99. doi: 10.4166/kjg.2016.67.6.289. Korean J Gastroenterol. 2016. PMID: 27312829 Review. Korean.
Cited by
- Intensive formation of coccoid forms as a feature strongly associated with highly pathogenic Helicobacter pylori strains.
Krzyżek P, Biernat MM, Gościniak G. Krzyżek P, et al. Folia Microbiol (Praha). 2019 May;64(3):273-281. doi: 10.1007/s12223-018-0665-5. Epub 2018 Nov 17. Folia Microbiol (Praha). 2019. PMID: 30449016 Free PMC article. - Challenges in Drug Discovery for Intracellular Bacteria.
Tucker AN, Carlson TJ, Sarkar A. Tucker AN, et al. Pathogens. 2021 Sep 11;10(9):1172. doi: 10.3390/pathogens10091172. Pathogens. 2021. PMID: 34578204 Free PMC article. - BabA-mediated adherence of pediatric ulcerogenic H. pylori strains to gastric mucins at neutral and acidic pH.
Quintana-Hayashi MP, Rocha R, Padra M, Thorell A, Jin C, Karlsson NG, Roxo-Rosa M, Oleastro M, Lindén SK. Quintana-Hayashi MP, et al. Virulence. 2018;9(1):1699-1717. doi: 10.1080/21505594.2018.1532243. Virulence. 2018. PMID: 30298790 Free PMC article. - Helicobacter pylori infection - recent developments in diagnosis.
Lopes AI, Vale FF, Oleastro M. Lopes AI, et al. World J Gastroenterol. 2014 Jul 28;20(28):9299-313. doi: 10.3748/wjg.v20.i28.9299. World J Gastroenterol. 2014. PMID: 25071324 Free PMC article. Review. - Proteomics As a Tool for Studying Bacterial Virulence and Antimicrobial Resistance.
Pérez-Llarena FJ, Bou G. Pérez-Llarena FJ, et al. Front Microbiol. 2016 Mar 31;7:410. doi: 10.3389/fmicb.2016.00410. eCollection 2016. Front Microbiol. 2016. PMID: 27065974 Free PMC article. Review.
References
- Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60:3–21. - PubMed
- Lai LH, Sung JJ. Helicobacter pylori and benign upper digestive disease. Best Pract Res Clin Gastroenterol. 2007;21:261–279. - PubMed
- Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63–96. - PubMed
- Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. - PubMed
- Walsh JH, Peterson WL. The treatment of Helicobacter pylori infection in the management of peptic ulcer disease. N Engl J Med. 1995;333:984–991. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources