Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study - PubMed (original) (raw)
Clinical Trial
Benefit from B-lymphocyte depletion using the anti-CD20 antibody rituximab in chronic fatigue syndrome. A double-blind and placebo-controlled study
Øystein Fluge et al. PLoS One. 2011.
Abstract
Background: Chronic fatigue syndrome (CFS) is a disease of unknown aetiology. Major CFS symptom relief during cancer chemotherapy in a patient with synchronous CFS and lymphoma spurred a pilot study of B-lymphocyte depletion using the anti-CD20 antibody Rituximab, which demonstrated significant clinical response in three CFS patients.
Methods and findings: In this double-blind, placebo-controlled phase II study (NCT00848692), 30 CFS patients were randomised to either Rituximab 500 mg/m(2) or saline, given twice two weeks apart, with follow-up for 12 months. Xenotropic murine leukemia virus-related virus (XMRV) was not detected in any of the patients. The responses generally affected all CFS symptoms. Major or moderate overall response, defined as lasting improvements in self-reported Fatigue score during follow-up, was seen in 10 out of 15 patients (67%) in the Rituximab group and in two out of 15 patients (13%) in the Placebo group (p = 0.003). Mean response duration within the follow-up period for the 10 responders to Rituximab was 25 weeks (range 8-44). Four Rituximab patients had clinical response durations past the study period. General linear models for repeated measures of Fatigue scores during follow-up showed a significant interaction between time and intervention group (p = 0.018 for self-reported, and p = 0.024 for physician-assessed), with differences between the Rituximab and Placebo groups between 6-10 months after intervention. The primary end-point, defined as effect on self-reported Fatigue score 3 months after intervention, was negative. There were no serious adverse events. Two patients in the Rituximab group with pre-existing psoriasis experienced moderate psoriasis worsening.
Conclusion: The delayed responses starting from 2-7 months after Rituximab treatment, in spite of rapid B-cell depletion, suggests that CFS is an autoimmune disease and may be consistent with the gradual elimination of autoantibodies preceding clinical responses. The present findings will impact future research efforts in CFS.
Trial registration: ClinicalTrials.gov NCT00848692.
Conflict of interest statement
Competing Interests: Haukeland University Hospital has patents and pending patent applications on the issue of B-cell depletion therapy for chronic fatigue syndrome. Family members of WO2009083602 A1 are pending, as well as granted US 12/348024. The two authors ØF and OM are named as inventors in these applications. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
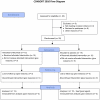
Figure 1. Study flow-diagram.
Approximately 60 patients with CFS diagnosed by a neurologist were identified from the hospital files and contacted by telephone for an interview, and 36 of these were invited for a further thorough assessment. During 12 months follow-up, one out of 15 patients in the placebo group was excluded from further analysis after 28 weeks due to pregnancy, and one after 42 weeks due to study withdrawal and patient's decision to start alternative therapy.
Figure 2. Fatigue scores in Rituximab and Placebo groups, self-reported and physician-assessed.
In panel A, the self-reported Fatigue scores were calculated for each patient every second week, from the mean of the four symptoms: Fatigue, Post-exertional exhaustion, Need for rest, Daily functioning. Then the mean values in Fatigue scores for the time intervals during follow-up were plotted. In panel C, the physician-assessed Fatigue scores were calculated from the mean of the same four symptoms, registered by the physician at the visits in the outpatient clinic. In panel B and D, estimated marginal means for self-reported and physician-assessed Fatigue scores during follow-up are shown. The scales on Y-axes were 0–6 (0: Major worsening; 1: Moderate worsening; 2: Slight worsening; 3: No change; 4: Slight improvement; 5: Moderate improvement; 6: Major improvement). The differences in distribution of Fatigue scores during follow-up, between the Rituximab and Placebo groups, were assessed by General Linear Model (GLM) for repeated measures, analysing the effects of time, the interaction time by intervention group, and the overall difference between intervention groups. Below panels C and D, the estimates for differences in mean Fatigue scores between the Rituximab and Placebo groups at the specific time intervals during follow-up, with 95% CI and p-values from the GLM (tests of within-subjects contrasts) are presented. In addition, Holm-Bonferroni step-down adjusted p-values for these time intervals are shown (five comparisons).
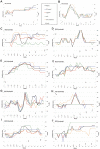
Figure 3. CFS symptom changes during follow-up for patients in the Rituximab group with significant responses.
In panels A–J, changes in Fatigue score (black), Cognitive score (red), Pain score (green), “Other symptoms” score (orange), and “CFS overall” score (blue), during 12 months follow-up are shown for the 10 patients in the Rituximab group with significant improvement. The scales on Y-axes were 0–6 (0: Major worsening; 1: Moderate worsening; 2: Slight worsening; 3: No change; 4: Slight improvement; 5: Moderate improvement; 6: Major improvement). Also shown are the B-cell numbers from immunophenotyping of peripheral blood mononuclear cells during follow-up (×106/L).
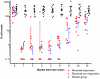
Figure 4. B-lymphocytes during follow-up.
B-cell numbers from immunophenotyping of peripheral blood during follow-up are shown, for patients in the Placebo group (black, n = 15), patients in the Rituximab group with significant response (red, n = 10), and patients in the Rituximab group with no response (blue, n = 5). The B-cell value zero was substituted by 0.1 (to be able to plot on the log scale). B-lymphocyte counts ×106/L (normal range 110–449). The error bars denote mean ± SEM.
Similar articles
- B-Lymphocyte Depletion in Myalgic Encephalopathy/ Chronic Fatigue Syndrome. An Open-Label Phase II Study with Rituximab Maintenance Treatment.
Fluge Ø, Risa K, Lunde S, Alme K, Rekeland IG, Sapkota D, Kristoffersen EK, Sørland K, Bruland O, Dahl O, Mella O. Fluge Ø, et al. PLoS One. 2015 Jul 1;10(7):e0129898. doi: 10.1371/journal.pone.0129898. eCollection 2015. PLoS One. 2015. PMID: 26132314 Free PMC article. Clinical Trial. - B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial.
Fluge Ø, Rekeland IG, Lien K, Thürmer H, Borchgrevink PC, Schäfer C, Sørland K, Aßmus J, Ktoridou-Valen I, Herder I, Gotaas ME, Kvammen Ø, Baranowska KA, Bohnen LMLJ, Martinsen SS, Lonar AE, Solvang AH, Gya AES, Bruland O, Risa K, Alme K, Dahl O, Mella O. Fluge Ø, et al. Ann Intern Med. 2019 May 7;170(9):585-593. doi: 10.7326/M18-1451. Epub 2019 Apr 2. Ann Intern Med. 2019. PMID: 30934066 Clinical Trial. - Clinical impact of B-cell depletion with the anti-CD20 antibody rituximab in chronic fatigue syndrome: a preliminary case series.
Fluge Ø, Mella O. Fluge Ø, et al. BMC Neurol. 2009 Jul 1;9:28. doi: 10.1186/1471-2377-9-28. BMC Neurol. 2009. PMID: 19566965 Free PMC article. - Anti-CD20 antibody in primary Sjögren's syndrome management.
Chen S, Liu Y, Shi G. Chen S, et al. Curr Pharm Biotechnol. 2014;15(6):535-41. doi: 10.2174/138920101506140910150431. Curr Pharm Biotechnol. 2014. PMID: 25213362 Review. - Rituximab for the treatment of fatigue in primary biliary cholangitis (formerly primary biliary cirrhosis): a randomised controlled trial.
Khanna A, Jopson L, Howel D, Bryant A, Blamire A, Newton JL, Wilkinson J, Steel AJ, Bainbridge J, Stefanetti R, Cassidy S, Houghton D, Jones DE. Khanna A, et al. Southampton (UK): NIHR Journals Library; 2018 Apr. Southampton (UK): NIHR Journals Library; 2018 Apr. PMID: 29733563 Free Books & Documents. Review.
Cited by
- CFS in Children and Adolescent: Ten Years of Retrospective Clinical Evaluation.
Elgen I, Hikmat O, Aspevik TN, Hagen EM. Elgen I, et al. Int J Pediatr. 2013;2013:270373. doi: 10.1155/2013/270373. Epub 2013 Jun 16. Int J Pediatr. 2013. PMID: 23843800 Free PMC article. - Chronic fatigue in patients with unexplained self-reported food hypersensitivity and irritable bowel syndrome: validation of a Norwegian translation of the Fatigue Impact Scale.
Lind R, Berstad A, Hatlebakk J, Valeur J. Lind R, et al. Clin Exp Gastroenterol. 2013 Jul 4;6:101-7. doi: 10.2147/CEG.S45760. Print 2013. Clin Exp Gastroenterol. 2013. PMID: 23869173 Free PMC article. - Antibody to Epstein-Barr virus deoxyuridine triphosphate nucleotidohydrolase and deoxyribonucleotide polymerase in a chronic fatigue syndrome subset.
Lerner AM, Ariza ME, Williams M, Jason L, Beqaj S, Fitzgerald JT, Lemeshow S, Glaser R. Lerner AM, et al. PLoS One. 2012;7(11):e47891. doi: 10.1371/journal.pone.0047891. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23155374 Free PMC article. - Current Research Provides Insight into the Biological Basis and Diagnostic Potential for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).
Sweetman E, Noble A, Edgar C, Mackay A, Helliwell A, Vallings R, Ryan M, Tate W. Sweetman E, et al. Diagnostics (Basel). 2019 Jul 10;9(3):73. doi: 10.3390/diagnostics9030073. Diagnostics (Basel). 2019. PMID: 31295930 Free PMC article. - Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: the biology of a neglected disease.
Arron HE, Marsh BD, Kell DB, Khan MA, Jaeger BR, Pretorius E. Arron HE, et al. Front Immunol. 2024 Jun 3;15:1386607. doi: 10.3389/fimmu.2024.1386607. eCollection 2024. Front Immunol. 2024. PMID: 38887284 Free PMC article. Review.
References
- Devanur LD, Kerr JR. Chronic fatigue syndrome. J Clin Virol. 2006;37:139–150. - PubMed
- Capelli E, Zola R, Lorusso L, Venturini L, Sardi F, et al. Chronic fatigue syndrome/myalgic encephalomyelitis: an update. Int J Immunopathol Pharmacol. 2010;23:981–989. - PubMed
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous