Brain-immune interactions and the neural basis of disease-avoidant ingestive behaviour - PubMed (original) (raw)
Review
Brain-immune interactions and the neural basis of disease-avoidant ingestive behaviour
Gustavo Pacheco-López et al. Philos Trans R Soc Lond B Biol Sci. 2011.
Abstract
Neuro-immune interactions are widely manifested in animal physiology. Since immunity competes for energy with other physiological functions, it is subject to a circadian trade-off between other energy-demanding processes, such as neural activity, locomotion and thermoregulation. When immunity is challenged, this trade-off is tilted to an adaptive energy protecting and reallocation strategy that is identified as 'sickness behaviour'. We review diverse disease-avoidant behaviours in the context of ingestion, indicating that several adaptive advantages have been acquired by animals (including humans) during phylogenetic evolution and by ontogenetic experiences: (i) preventing waste of energy by reducing appetite and consequently foraging/hunting (illness anorexia), (ii) avoiding unnecessary danger by promoting safe environments (preventing disease encounter by olfactory cues and illness potentiation neophobia), (iii) help fighting against pathogenic threats (hyperthermia/somnolence), and (iv) by associative learning evading specific foods or environments signalling danger (conditioned taste avoidance/aversion) and/or at the same time preparing the body to counteract by anticipatory immune responses (conditioning immunomodulation). The neurobiology behind disease-avoidant ingestive behaviours is reviewed with special emphasis on the body energy balance (intake versus expenditure) and an evolutionary psychology perspective.
Figures
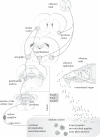
Figure 1.
Appetitive and consummatory behaviours are significantly affected by the immune-derived molecules elicited by peripheral immune challenge resulting in innate and learned disease-avoidant ingestive behaviours. Such avoidant behaviours are the result of phylogenetic evolution and ontogenetic experiences with the ultimate goals of: (i) preventing waste of energy, (ii) avoiding unnecessary danger by promoting safe environments, (iii) helping to fight against pathogenic threats, and (iv) by associative learning it may also be possible to evade specific foods or environments signalling danger and/or at the same time prepare the body to counteract by anticipatory immune responses.
Figure 2.
Neurobiology of disease-avoidant ingestive behaviours. The vagus nerve provides the major neural pathway identified to date. From bottom to top, the initial chemosensory transduction events occur in immune cells that respond to specific chemical components expressed by dangerous micro-organisms. Afterwards in a paracrine-like manner, a close interaction of lymphocytes with sensory neurons bearing appropriate immune-transmitter receptors (e.g. cytokines, prostaglandins, neurotransmitters) leads to viscera–sensory afferent neural signalling [24,30]. This neural pathway is complemented by a humoral afferent pathway (dotted line) involving access of immune-generated molecules throughout circumventricular organs and/or being transduced within the brain perivascular space (see figure 3). In general, the hypothalamus and the nucleus of the solitary tract are constantly involved in processing visceral–immune signalling, being two key neural integrators of energy homeostasis (e.g. appetite, thermoregulation, sleep; reproduction [31]). Besides these structures, two cortico-limbic structures are of relevance within neuro–immune interactions; the insular cortex and the amygdala, being neural nuclei responsible for: (i) stimuli hedonic categorization, (ii) emotionality, and (iii) associative learning, in animals as well as in humans [–36]. Another important sensory route used to avoid sick individuals or contaminated food is the olfactory system. Specific vomeronasal receptors are capable of detecting volatile molecules derived from activated leucocytes and also from pathogens [37]. From top to bottom, this sensory activation would modulate behaviour through canonical olfactory relays in the olfactory bulb, pyriniform cortex and hypothalamus.
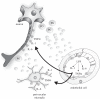
Figure 3.
Pericerebral neuro–immune interactions. Immune-transmitters can cross the blood–brain barrier or originate a second wave of diffusible messengers within the brain parenchyma [26,38,39]. Cytokines or prostaglandin locally produced by endothelial cells or perivascular microglia can activate neurons that project to specific brain areas or diffuse into the brain parenchyma to reach their targets. Thus, activation of neurons can be direct or indirect and potentially occurs in the whole brain. However, receptors relevant for neuro–immune communication in the brain are preferentially located in the vicinity of circumventricular organs, from which the signal is relayed to other brain regions through neuron-to-neuron comunication. IL, interleukin; LPS, lipopolysaccharide; PG, prostaglandins; TNF, tumour necrosis factor.
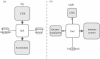
Figure 4.
Circadian fuel allocation. During the day (a) many aspects of immune function are inhibited due to the immunosuppressive effects of cortisol and noradrenaline. In contrast at midnight (b), the level of these hormones is low, but growth hormone, prolactin and melatonin are secreted shortly after sleep onset, which activates the immune cells [10]. A tight circadian allocation of fuel is accomplished to cover energy demands optimally. However, when immunity is compromised an ‘_energy demand reaction_’ is elicited by leucocytes mobilizing fuel stocks and supressing demand from other organs/systems, with the overall purpose of preferentially allocating fuels to activated immune cells. Concomitant behavioural changes prevent waste of energy, avoid unnecessary danger and promote safe environments, but at the same time help fight against pathogenic threats; if they survived, the individual would probably form associative memories to anticipate similar threats and/or to counteract inevitable future encounters.
Similar articles
- Overconsumption as a cause of weight gain: behavioural-physiological interactions in the control of food intake (appetite).
Blundell JE, King NA. Blundell JE, et al. Ciba Found Symp. 1996;201:138-54; discussion 154-8, 188-93. doi: 10.1002/9780470514962.ch9. Ciba Found Symp. 1996. PMID: 9017279 Review. - Behavioral regulation of the milieu interne in man and rat.
Garcia J, Hankins WG, Rusiniak KW. Garcia J, et al. Science. 1974 Sep 6;185(4154):824-31. doi: 10.1126/science.185.4154.824. Science. 1974. PMID: 11785521 - Interactions of temperature and taste in conditioned aversions.
Smith PL, Smith JC, Houpt TA. Smith PL, et al. Physiol Behav. 2010 Mar 3;99(3):324-33. doi: 10.1016/j.physbeh.2009.11.008. Epub 2009 Nov 26. Physiol Behav. 2010. PMID: 19945474 Free PMC article. - Odors can induce feeding motor responses in the terrestrial mollusc Limax maximus.
Sahley CL, Martin KA, Gelperin A. Sahley CL, et al. Behav Neurosci. 1992 Jun;106(3):563-8. doi: 10.1037//0735-7044.106.3.563. Behav Neurosci. 1992. PMID: 1616620 - Appetite and energy balancing.
Rogers PJ, Brunstrom JM. Rogers PJ, et al. Physiol Behav. 2016 Oct 1;164(Pt B):465-471. doi: 10.1016/j.physbeh.2016.03.038. Epub 2016 Apr 6. Physiol Behav. 2016. PMID: 27059321 Review.
Cited by
- Interoception and Inflammation in Psychiatric Disorders.
Savitz J, Harrison NA. Savitz J, et al. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018 Jun;3(6):514-524. doi: 10.1016/j.bpsc.2017.12.011. Epub 2018 Jan 9. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018. PMID: 29884282 Free PMC article. Review. - Aberrant disgust responses and immune reactivity in cocaine-dependent men.
Ersche KD, Hagan CC, Smith DG, Abbott S, Jones PS, Apergis-Schoute AM, Döffinger R. Ersche KD, et al. Biol Psychiatry. 2014 Jan 15;75(2):140-7. doi: 10.1016/j.biopsych.2013.08.004. Epub 2013 Oct 3. Biol Psychiatry. 2014. PMID: 24090796 Free PMC article. - Peripheral and central compensatory mechanisms for impaired vagus nerve function during peripheral immune activation.
Kobrzycka A, Napora P, Pearson BL, Pierzchała-Koziec K, Szewczyk R, Wieczorek M. Kobrzycka A, et al. J Neuroinflammation. 2019 Jul 19;16(1):150. doi: 10.1186/s12974-019-1544-y. J Neuroinflammation. 2019. PMID: 31324250 Free PMC article. - Neuronal basis and diverse mechanisms of pathogen avoidance in Caenorhabditis elegans.
Lei M, Tan Y, Tu H, Tan W. Lei M, et al. Front Immunol. 2024 May 1;15:1353747. doi: 10.3389/fimmu.2024.1353747. eCollection 2024. Front Immunol. 2024. PMID: 38751431 Free PMC article. Review. - Microglial Morphology Across Distantly Related Species: Phylogenetic, Environmental and Age Influences on Microglia Reactivity and Surveillance States.
Carvalho-Paulo D, Bento Torres Neto J, Filho CS, de Oliveira TCG, de Sousa AA, Dos Reis RR, Dos Santos ZA, de Lima CM, de Oliveira MA, Said NM, Freitas SF, Sosthenes MCK, Gomes GF, Henrique EP, Pereira PDC, de Siqueira LS, de Melo MAD, Guerreiro Diniz C, Magalhães NGM, Diniz JAP, Vasconcelos PFDC, Diniz DG, Anthony DC, Sherry DF, Brites D, Picanço Diniz CW. Carvalho-Paulo D, et al. Front Immunol. 2021 Jun 18;12:683026. doi: 10.3389/fimmu.2021.683026. eCollection 2021. Front Immunol. 2021. PMID: 34220831 Free PMC article. Review.
References
- Ader R., Cohen N., Felten D. 1995. Psychoneuroimmunology: interactions between the nervous system and the immune system. Lancet 345, 99–10310.1016/S0140-6736(95)90066-7 (doi:10.1016/S0140-6736(95)90066-7) - DOI - DOI - PubMed
- Salzet M. 2000. Invertebrate molecular neuroimmune processes. Brain Res. Brain Res. Rev. 34, 69–7910.1016/S0165-0173(00)00041-2 (doi:10.1016/S0165-0173(00)00041-2) - DOI - DOI - PubMed
- Ottaviani E., Franceschi C. 1996. The neuroimmunology of stress from invertebrates to man. Prog. Neurobiol. 48, 421–44010.1016/0301-0082(95)00049-6 (doi:10.1016/0301-0082(95)00049-6) - DOI - DOI - PubMed
- Farr M., Zhu D. F., Povelones M., Valcich D., Ambron R. T. 2001. Direct interactions between immunocytes and neurons after axotomy in aplysia. J. Neurobiol. 46, 89–9610.1002/1097-4695(20010205)46:2<89::AID-NEU20>3.0.CO;2-D (doi:10.1002/1097-4695(20010205)46:2<89::AID-NEU20>3.0.CO;2-D) - DOI - DOI - PubMed
- Mallon E. B., Brockmann A., Schmid-Hempel P. 2003. Immune response inhibits associative learning in insects. Proc. R. Soc. Lond. B 270, 2471–247310.1098/rspb.2003.2456 (doi:10.1098/rspb.2003.2456) - DOI - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical