Tau-targeted treatment strategies in Alzheimer's disease - PubMed (original) (raw)
Review
Tau-targeted treatment strategies in Alzheimer's disease
Jürgen Götz et al. Br J Pharmacol. 2012 Mar.
Abstract
With populations ageing worldwide, the need for treating and preventing diseases associated with high age is pertinent. Alzheimer's disease (AD) is reaching epidemic proportions, yet the currently available therapies are limited to a symptomatic relief, without halting the degenerative process that characterizes the AD brain. As in AD cholinergic neurons are lost at high numbers, the initial strategies were limited to the development of acetylcholinesterase inhibitors, and more recently the NMDA receptor antagonist memantine, in counteracting excitotoxicity. With the identification of the protein tau in intracellular neurofibrillary tangles and of the peptide amyloid-β (Aβ) in extracellular amyloid plaques in the AD brain, and a better understanding of their role in disease, newer strategies are emerging, which aim at either preventing their formation and deposition or at accelerating their clearance. Interestingly, what is well established to combat viral diseases in peripheral organs - vaccination - seems to work for the brain as well. Accordingly, immunization strategies targeting Aβ show efficacy in mice and to some degree also in humans. Even more surprising is the finding in mice that immunization strategies targeting tau, a protein that forms aggregates in nerve cells, ameliorates the tau-associated pathology. We are reviewing the literature and discuss what can be expected regarding the translation into clinical practice and how the findings can be extended to other neurodegenerative diseases with protein aggregation in brain.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures
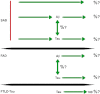
Figure 1
Relative contribution of the key players in AD and FTLD-Tau in toxicity. What causes SAD is not known. Most likely neuronal dysfunction and the loss of neurons are initiated by a range of triggers, such as toxins or oxidative stress that use Aβ, tau or an unknown mediator in executing their toxic functions. Aβ and tau dysregulation have direct consequences on neuronal function. There is also a significant crosstalk between Aβ and tau, in that Aβ is upstream of tau (as formulated by the amyloid cascade hypothesis), but at the same time Aβ toxicity is tau-dependent. For the rare FAD cases, the situation is more defined in that the known FAD mutations (that are all localized in the APP, PSEN1 and PSEN2 gene, respectively) are linked to Aβ formation, but again there is a crosstalk between Aβ and tau. Finally, in FTLD-Tau, tau dysfunction and NFT formation occur in the absence of a contribution of Aβ. A central question in the field and important as regards treatment strategies is what the relative contribution (%) of the Aβ-and tau-dependent as well as -independent mechanisms are in AD. This is also relevant (see subsequent figures) for the cellular compartments in which tau and Aβ exert their toxic functions and the cellular mechanisms (such as transport, signal transduction or mitochondrial function) they are believed to impair.
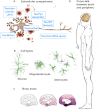
Figure 2
The compartmentalization of tau as a key player. (A) Tau's established physiological function is in the axon, where it is bound to and believed to stabilize microtubules. We found recently that under physiological conditions tau is also in dendritic spines where it has a function in targeting the PSD95 kinase Fyn to the post-synaptic density. In addition, tau has a role in the nucleus, either directly or indirectly, by altering the nucleo-cytoplasmic distribution of splicing factors. Pathological tau affects mitochondrial functions, specifically by targeting complex I of the mitochondrial respiratory chain. Furthermore, under pathological conditions, as tau is re-localized from the axon to the somatodendritic domain, it traps proteins (such as Jip1) in the soma and prevents them from executing their physiological function in the axonal compartment. Finally, new evidence suggests that tau is secreted into the interstitial space, potentially allowing for removal of interstitial tau by tau-targeted vaccination approaches. (B) An additional layer of complexity is added by the finding that although tau is perceived as a neuronal protein, under pathological conditions, such as in PSP or in CBD, tau forms fibrillar aggregates in astrocytes and oligodendrocytes. (C) As AD progresses, tau spreads in a stereotyped fashion through the brain. This observation has led to the definition of the so-called Braak stages. It can be envisaged that tau's toxic functions vary depending on the brain area where tau accumulates. (D) There is an increasing appreciation that the periphery, and in particular the immune system, has a decisive role, in a cross-talk with the brain, in the AD pathology.
Figure 3
Tau-targeted therapeutic strategies. A host of strategies have been devised in tau-transgenic animal models to ameliorate biochemical changes due to tau over-expression (such as insolubility and hyperphosphorylation), histological alterations (such as NFT formation and somatodendritic localization) as well as behavioural (both memory and motor) impairments. To combat AD rather than only targeting Aβ, a combinatorial tau/Aβ strategy is likely the better approach.
Similar articles
- Immunotherapy for Alzheimer's disease.
Wang W, Fan L, Xu D, Wen Z, Yu R, Ma Q. Wang W, et al. Acta Biochim Biophys Sin (Shanghai). 2012 Oct;44(10):807-14. doi: 10.1093/abbs/gms065. Epub 2012 Aug 16. Acta Biochim Biophys Sin (Shanghai). 2012. PMID: 22899646 Review. - Active full-length DNA Aβ42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology.
Rosenberg RN, Fu M, Lambracht-Washington D. Rosenberg RN, et al. Alzheimers Res Ther. 2018 Nov 20;10(1):115. doi: 10.1186/s13195-018-0441-4. Alzheimers Res Ther. 2018. PMID: 30454039 Free PMC article. - Immunotherapy for Alzheimer's disease: from anti-β-amyloid to tau-based immunization strategies.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A, Greco A, Seripa D, Pilotto A. Panza F, et al. Immunotherapy. 2012 Feb;4(2):213-38. doi: 10.2217/imt.11.170. Immunotherapy. 2012. PMID: 22339463 Review. - Tau-based therapeutic approaches for Alzheimer's disease - a mini-review.
Boutajangout A, Wisniewski T. Boutajangout A, et al. Gerontology. 2014;60(5):381-5. doi: 10.1159/000358875. Epub 2014 Apr 8. Gerontology. 2014. PMID: 24732638 Free PMC article. Review. - Immunotherapeutic approaches for Alzheimer's disease.
Wisniewski T, Goñi F. Wisniewski T, et al. Neuron. 2015 Mar 18;85(6):1162-76. doi: 10.1016/j.neuron.2014.12.064. Neuron. 2015. PMID: 25789753 Free PMC article. Review.
Cited by
- Genome instability in Alzheimer disease.
Hou Y, Song H, Croteau DL, Akbari M, Bohr VA. Hou Y, et al. Mech Ageing Dev. 2017 Jan;161(Pt A):83-94. doi: 10.1016/j.mad.2016.04.005. Epub 2016 Apr 20. Mech Ageing Dev. 2017. PMID: 27105872 Free PMC article. Review. - Biomarkers in amyloid-β immunotherapy trials in Alzheimer's disease.
Blennow K, Hampel H, Zetterberg H. Blennow K, et al. Neuropsychopharmacology. 2014 Jan;39(1):189-201. doi: 10.1038/npp.2013.154. Epub 2013 Jun 25. Neuropsychopharmacology. 2014. PMID: 23799530 Free PMC article. Review. - Alzheimer disease therapy--moving from amyloid-β to tau.
Giacobini E, Gold G. Giacobini E, et al. Nat Rev Neurol. 2013 Dec;9(12):677-86. doi: 10.1038/nrneurol.2013.223. Epub 2013 Nov 12. Nat Rev Neurol. 2013. PMID: 24217510 Review. - Connecting the dots between tau dysfunction and neurodegeneration.
Frost B, Götz J, Feany MB. Frost B, et al. Trends Cell Biol. 2015 Jan;25(1):46-53. doi: 10.1016/j.tcb.2014.07.005. Epub 2014 Aug 26. Trends Cell Biol. 2015. PMID: 25172552 Free PMC article. Review. - Immunotherapeutic Approaches Targeting Amyloid-β, α-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders.
Valera E, Spencer B, Masliah E. Valera E, et al. Neurotherapeutics. 2016 Jan;13(1):179-89. doi: 10.1007/s13311-015-0397-z. Neurotherapeutics. 2016. PMID: 26494242 Free PMC article. Review.
References
- Agarwal-Mawal A, Qureshi HY, Cafferty PW, Yuan Z, Han D, Lin R, et al. 14-3-3 connects glycogen synthase kinase-3 beta to tau within a brain microtubule-associated tau phosphorylation complex. J Biol Chem. 2003;278:12722–12728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials