Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities - PubMed (original) (raw)
Clinical Trial
Randomized, double-blind, placebo-controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities
Mike M Nguyen et al. Cancer Prev Res (Phila). 2012 Feb.
Abstract
Compelling preclinical and pilot clinical data support the role of green tea polyphenols in prostate cancer prevention. We conducted a randomized, double-blind, placebo-controlled trial of polyphenon E (enriched green tea polyphenol extract) in men with prostate cancer scheduled to undergo radical prostatectomy. The study aimed to determine the bioavailability of green tea polyphenols in prostate tissue and to measure its effects on systemic and tissue biomarkers of prostate cancer carcinogenesis. Participants received either polyphenon E (containing 800 mg epigallocatechin gallate) or placebo daily for 3 to 6 weeks before surgery. Following the intervention, green tea polyphenol levels in the prostatectomy tissue were low to undetectable. Polyphenon E intervention resulted in favorable but not statistically significant changes in serum prostate-specific antigen, serum insulin-like growth factor axis, and oxidative DNA damage in blood leukocytes. Tissue biomarkers of cell proliferation, apoptosis, and angiogenesis in the prostatectomy tissue did not differ between the treatment arms. The proportion of subjects who had a decrease in Gleason score between biopsy and surgical specimens was greater in those on polyphenon E but was not statistically significant. The study's findings of low bioavailability and/or bioaccumulation of green tea polyphenols in prostate tissue and statistically insignificant changes in systemic and tissue biomarkers from 3 to 6 weeks of administration suggests that prostate cancer preventive activity of green tea polyphenols, if occurring, may be through indirect means and/or that the activity may need to be evaluated with longer intervention durations, repeated dosing, or in patients at earlier stages of the disease.
Trial registration: ClinicalTrials.gov NCT00459407.
©2011 AACR.
Figures
Figure 1
Consort flow diagram.
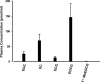
Figure 2
Post-intervention plasma green tea polyphenol concentrations following 3–6 weeks of Polyphenon E intervention. EGC: epigallocatechin; EC: epicatechin; ECG: epicatechin gallate; EGCG: epigallocatechin gallate; 4″-MeEGCG: 4″-O-methyl EGCG. Data are presented as means with standard errors (n = 24).
Similar articles
- Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals.
Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Chow HH, et al. Clin Cancer Res. 2003 Aug 15;9(9):3312-9. Clin Cancer Res. 2003. PMID: 12960117 Clinical Trial. - Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals.
Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Chow HH, et al. Clin Cancer Res. 2005 Jun 15;11(12):4627-33. doi: 10.1158/1078-0432.CCR-04-2549. Clin Cancer Res. 2005. PMID: 15958649 Clinical Trial. - Results of a phase II randomized, double-blind, placebo-controlled trial of Polyphenon E in women with persistent high-risk HPV infection and low-grade cervical intraepithelial neoplasia.
Garcia FA, Cornelison T, Nuño T, Greenspan DL, Byron JW, Hsu CH, Alberts DS, Chow HH. Garcia FA, et al. Gynecol Oncol. 2014 Feb;132(2):377-82. doi: 10.1016/j.ygyno.2013.12.034. Epub 2014 Jan 2. Gynecol Oncol. 2014. PMID: 24388920 Free PMC article. Clinical Trial. - Green tea polyphenols for prostate cancer chemoprevention: a translational perspective.
Johnson JJ, Bailey HH, Mukhtar H. Johnson JJ, et al. Phytomedicine. 2010 Jan;17(1):3-13. doi: 10.1016/j.phymed.2009.09.011. Phytomedicine. 2010. PMID: 19959000 Free PMC article. Review. - Chemoprevention of nonmelanoma skin cancer: experience with a polyphenol from green tea.
Linden KG, Carpenter PM, McLaren CE, Barr RJ, Hite P, Sun JD, Li KT, Viner JL, Meyskens FL. Linden KG, et al. Recent Results Cancer Res. 2003;163:165-71; discussion 264-6. doi: 10.1007/978-3-642-55647-0_15. Recent Results Cancer Res. 2003. PMID: 12903852 Review.
Cited by
- Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials.
Isomura T, Suzuki S, Origasa H, Hosono A, Suzuki M, Sawada T, Terao S, Muto Y, Koga T. Isomura T, et al. Eur J Clin Nutr. 2016 Nov;70(11):1221-1229. doi: 10.1038/ejcn.2016.78. Epub 2016 May 18. Eur J Clin Nutr. 2016. PMID: 27188915 Free PMC article. Review. - Biological effects of green tea capsule supplementation in pre-surgery postmenopausal breast cancer patients.
Yu SS, Spicer DV, Hawes D, Tseng CC, Yang CS, Pike MC, Wu AH. Yu SS, et al. Front Oncol. 2013 Dec 13;3:298. doi: 10.3389/fonc.2013.00298. eCollection 2013. Front Oncol. 2013. PMID: 24380073 Free PMC article. - Green Tea Catechins for Prostate Cancer Prevention: Present Achievements and Future Challenges.
Naponelli V, Ramazzina I, Lenzi C, Bettuzzi S, Rizzi F. Naponelli V, et al. Antioxidants (Basel). 2017 Apr 5;6(2):26. doi: 10.3390/antiox6020026. Antioxidants (Basel). 2017. PMID: 28379200 Free PMC article. Review. - Neurodegenerative diseases and catechins: (-)-epigallocatechin-3-gallate is a modulator of chronic neuroinflammation and oxidative stress.
Li S, Wang Z, Liu G, Chen M. Li S, et al. Front Nutr. 2024 Aug 1;11:1425839. doi: 10.3389/fnut.2024.1425839. eCollection 2024. Front Nutr. 2024. PMID: 39149548 Free PMC article. Review. - Angioprevention of Urologic Cancers by Plant-Derived Foods.
García-Caballero M, Torres-Vargas JA, Marrero AD, Martínez-Poveda B, Medina MÁ, Quesada AR. García-Caballero M, et al. Pharmaceutics. 2022 Jan 21;14(2):256. doi: 10.3390/pharmaceutics14020256. Pharmaceutics. 2022. PMID: 35213989 Free PMC article. Review.
References
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349:215–24. - PubMed
- Andriole GL, Bostwick DG, Brawley OW, Gomella LG, Marberger M, Montorsi F, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362:1192–202. - PubMed
- Hamilton RJ, Kahwati LC, Kinsinger LS. Knowledge and use of finasteride for the prevention of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2010;19:2164–71. - PubMed
- Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical