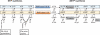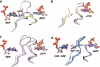DNA building blocks: keeping control of manufacture - PubMed (original) (raw)
Review
DNA building blocks: keeping control of manufacture
Anders Hofer et al. Crit Rev Biochem Mol Biol. 2012 Jan-Feb.
Abstract
Ribonucleotide reductase (RNR) is the only source for de novo production of the four deoxyribonucleoside triphosphate (dNTP) building blocks needed for DNA synthesis and repair. It is crucial that these dNTP pools are carefully balanced, since mutation rates increase when dNTP levels are either unbalanced or elevated. RNR is the major player in this homeostasis, and with its four different substrates, four different allosteric effectors and two different effector binding sites, it has one of the most sophisticated allosteric regulations known today. In the past few years, the structures of RNRs from several bacteria, yeast and man have been determined in the presence of allosteric effectors and substrates, revealing new information about the mechanisms behind the allosteric regulation. A common theme for all studied RNRs is a flexible loop that mediates modulatory effects from the allosteric specificity site (s-site) to the catalytic site for discrimination between the four substrates. Much less is known about the allosteric activity site (a-site), which functions as an on-off switch for the enzyme's overall activity by binding ATP (activator) or dATP (inhibitor). The two nucleotides induce formation of different enzyme oligomers, and a recent structure of a dATP-inhibited α(6)β(2) complex from yeast suggested how its subunits interacted non-productively. Interestingly, the oligomers formed and the details of their allosteric regulation differ between eukaryotes and Escherichia coli. Nevertheless, these differences serve a common purpose in an essential enzyme whose allosteric regulation might date back to the era when the molecular mechanisms behind the central dogma evolved.
Figures
Figure 1
NTP and dNTP synthesis with allosterically/feedback regulated enzymes in dNTP synthesis shown. The class of RNR used, the presence of different dNKs and the choice of dCMP or dCTP for deamination vary between species. Most eukaryotes (e.g. mammals) and gram positive bacteria use dCMP deaminase (dCMPDA) whereas most gram-negative bacteria use dCTP deaminase (dCTPDA). The one-letter abbreviations in NTP salvage synthesis (U, C, A, G and I) stand for both the base and its corresponding ribonucleoside (I stands for hypoxanthine and inosine). Degradation pathways are generally not included with the exception of dephosphorylation events, which are shown by grey arrows unless they directly take part in dNTP synthesis. The dephosphorylations of (d)NTPs to (d)NMPs can both occur as a one-step procedure or via (d)NDPs.
Figure 2
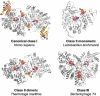
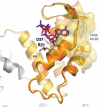
Structures of catalytic subunits from different RNR classes with bound nucleotides highlighted. The a-sites, s-sites, catalytic sites and loop 2 are indicated by the labels a, s, c and 2, respectively. The ATP cones in each α subunit of the human class I enzyme are shown in orange or gold, respectively, whereas it is absent in the representatives from the other classes. Loop 1 and loop 2 are coloured in yellow and red, respectively.
Figure 3
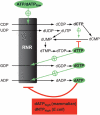
Overview of the allosteric regulation of de novo dNTP synthesis. RNR is regulated by four different allosteric effectors (highlighted) and has both a specificity regulation that determines what substrate to reduce (arrows pointed towards RNR) and an overall activity regulation that can turn the enzyme off when there is no need to synthesize more dNTPs (bottom box). Also included in the scheme is dCMP deaminase, which controls the concentration ratio between dCTP and dTTP (the organisms with dCTP deaminase have a similar regulation). Recently, it has been realized that Nature has chosen two different strategies to achieve the overall regulation (bottom box). The E. coli enzyme is turned off by high dNTP/ATP ratios (dNTP = dATP, dTTP or dGTP), whereas the mammalian enzyme can only be turned off by high dATP/ATP ratios.
Figure 4
Structural basis for allosteric substrate specificity regulation, (a) The effect of different substrate-effector pairs on loop 2 structure in the T. maritima class II enzyme. Nucleotide carbon atoms and loop 2 are coloured grey for the dTTP/GDP complex, yellow for dGTP/ ADP, pink for dATP/CDP and light blue for dATP/UDP. The missing parts of loop 2 in the pink, blue and yellow structures were not visible in the crystal structures and represent unstructured elements. Note in particular the fully ordered loop 2 in dTTP/GDP and the projection towards the substrate of two different hydrogen-bonding residues, K202 and Q203, in dTTP/GDP and dATP/CDP(UDP), respectively, (b-c) Generality across species and RNR classes of the cooperative effect in the binding of dTTP and GDP. The complexes from T. maritima class II RNR are coloured grey, S. cerevisiae class la in pink, human in light blue and S. typhimurium class Ib in yellow (note that there is no dTTP/GDP complex available for S. typhimurium). In the absence of substrate, dTTP is unable to structure loop 2 (b) but in its presence (c), loop 2 becomes ordered and forms a cradle around the guanine base of GDP. Only main chain atoms and water molecules are involved in substrate base recognition, (d) Common features of specificity regulation also extend to the dATP/CDP and dATP/UDP complexes shown in light and dark grey, respectively, for T. maritima, and light and dark blue respectively for S. cerevisiae. Note that a glutamine residue (Q203 in T. maritima, Q288 in S. cerevisiae) from the effector-proximal side of loop 2 is always projected towards the substrate.
Figure 5
Structure of the ATP cone domain of human class I RNR in complex with dATP (blue) and ATP (red). Residue Asp57, which when mutated eliminates ability to discriminate between ATP and dATP, is shown in stick representation, as is one of its salt bridge partners, Arg21. The helices are labeled H1-H4. The residues involved in the interactions between three α dimers in the dATP-inhibited oligomer (α6β2 complex) as determined by electron microscopy are shown as a gold surface. The largest differences between the two complexes are in the loop 45-52, which is an important component of the dimer-dimer interface.
Figure 6
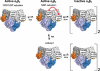
Overall activity regulation in class I RNRs. General for this class is that all allosteric effectors are able to stimulate the formation of α dimers and active α2β2 complexes. In species with overall activity regulation, heavier complexes are formed in a species-dependent manner. In eukaryotes (mammals and S. cerevisiae), the α subunit can form a hexamer that interacts with the β2 subunit to form an inactive α6β2 or fully active α6β2-6 complex (higher activity than α2β2) depending on whether dATP or ATP is bound to the a-site. In E. coli, the α2β2 complexes can bind to each other in the presence of dATP or effector combinations of ATP and high concentrations of dNTPs and thereby form an inactive α4β4 complex. The protein structures shown are α/α2 (human), α6 (S. cerevisiae), α6β2 α6(S. cerevisiae, based on cryo-EM structure) and a model of the α6β2 complex from E. coli built from separate structures of α2and β2(Uhlin and Eklund, 1994).
Figure 7
The s-site has a key role in overall activity regulation of the E. coli RNR. If the s-site is occupied by a dNTP, a cross-talk signal between the nucleotides in the two allosteric sites (double-sided arrow) leads to the formation of an inhibited α4β4 complex. At low dNTP concentrations, ATP is able to compete for the s-site and the enzyme rapidly equilibrates between the two top left forms in the figure. At higher dNTP concentrations, the concentration of the form shown in the top middle is high enough to promote formation of the inactive α4β4 complex (Rofougaran et al., 2008). The lower part of the picture shows that the inhibited form is also formed with dATP in the a-site. However, in this case it is not known whether the intermediate α2β2 form is active and if a cross-talk signal between the two sites is required for inhibition. Theoretically, a form with dATP in the a-site and ATP in the s-site is also conceivable, but is excluded from the scheme since it is uncertain if ATP ever can compete significantly with binding to the s-site when the dATP concentration is high enough to bind to both sites.
Similar articles
- Diversity in Overall Activity Regulation of Ribonucleotide Reductase.
Jonna VR, Crona M, Rofougaran R, Lundin D, Johansson S, Brännström K, Sjöberg BM, Hofer A. Jonna VR, et al. J Biol Chem. 2015 Jul 10;290(28):17339-48. doi: 10.1074/jbc.M115.649624. Epub 2015 May 13. J Biol Chem. 2015. PMID: 25971975 Free PMC article. - Allosteric regulation of the class III anaerobic ribonucleotide reductase from bacteriophage T4.
Andersson J, Westman M, Hofer A, Sjoberg BM. Andersson J, et al. J Biol Chem. 2000 Jun 30;275(26):19443-8. doi: 10.1074/jbc.M001490200. J Biol Chem. 2000. PMID: 10748029 - Basis of dATP inhibition of RNRs.
Greene BL, Nocera DG, Stubbe J. Greene BL, et al. J Biol Chem. 2018 Jun 29;293(26):10413-10414. doi: 10.1074/jbc.H118.003717. J Biol Chem. 2018. PMID: 29959279 Free PMC article. - The structural basis for the allosteric regulation of ribonucleotide reductase.
Ahmad MF, Dealwis CG. Ahmad MF, et al. Prog Mol Biol Transl Sci. 2013;117:389-410. doi: 10.1016/B978-0-12-386931-9.00014-3. Prog Mol Biol Transl Sci. 2013. PMID: 23663976 Free PMC article. Review. - The prototypic class Ia ribonucleotide reductase from Escherichia coli: still surprising after all these years.
Brignole EJ, Ando N, Zimanyi CM, Drennan CL. Brignole EJ, et al. Biochem Soc Trans. 2012 Jun 1;40(3):523-30. doi: 10.1042/BST20120081. Biochem Soc Trans. 2012. PMID: 22616862 Free PMC article. Review.
Cited by
- Conformational control over proton-coupled electron transfer in metalloenzymes.
Fatima S, Olshansky L. Fatima S, et al. Nat Rev Chem. 2024 Oct;8(10):762-775. doi: 10.1038/s41570-024-00646-7. Epub 2024 Sep 2. Nat Rev Chem. 2024. PMID: 39223400 Free PMC article. Review. - Nucleotide binding to the ATP-cone in anaerobic ribonucleotide reductases allosterically regulates activity by modulating substrate binding.
Bimai O, Banerjee I, Rozman Grinberg I, Huang P, Hultgren L, Ekström S, Lundin D, Sjöberg BM, Logan DT. Bimai O, et al. Elife. 2024 Jul 5;12:RP89292. doi: 10.7554/eLife.89292. Elife. 2024. PMID: 38968292 Free PMC article. - Conserved _C_-Terminal Tail Is Responsible for Membrane Localization and Function of Pseudomonas aeruginosa Hemerythrin.
Stuut Balsam S, Zhong F, Pence N, Levintov L, Andhare D, Hammond JH, Ragusa MJ, Vashisth H, Hogan DA, Pletneva EV. Stuut Balsam S, et al. Biochemistry. 2024 Jul 16;63(14):1795-1807. doi: 10.1021/acs.biochem.4c00174. Epub 2024 Jul 1. Biochemistry. 2024. PMID: 38951132 Free PMC article. - Physical interactions between specifically regulated subpopulations of the MCM and RNR complexes prevent genetic instability.
Yáñez-Vilches A, Romero AM, Barrientos-Moreno M, Cruz E, González-Prieto R, Sharma S, Vertegaal ACO, Prado F. Yáñez-Vilches A, et al. PLoS Genet. 2024 May 22;20(5):e1011148. doi: 10.1371/journal.pgen.1011148. eCollection 2024 May. PLoS Genet. 2024. PMID: 38776358 Free PMC article. - A deoxynucleoside triphosphate triphosphohydrolase promotes cell cycle progression in Caulobacter crescentus.
Hellenbrand CN, Stevenson DM, Gromek KA, Amador-Noguez D, Hershey DM. Hellenbrand CN, et al. bioRxiv [Preprint]. 2024 Apr 26:2024.04.25.591158. doi: 10.1101/2024.04.25.591158. bioRxiv. 2024. PMID: 38712277 Free PMC article. Preprint.
References
- Andersson J, Westman M, Hofer A, Sjöberg BM. Allosteric regulation of the class III anaerobic ribonucleotide reductase from bacteriophage T4. J Biol Chem. 2000;275:19443–19448. - PubMed
- Aravind L, Wolf YI, Koonin EV. The ATP-cone: an evolutionarily mobile, ATP-binding regulatory domain. J Mol Microbiol Biotechnol. 2000;2:191–194. - PubMed
- Averett DR, Lubbers C, Elion GB, Spector T. Ribonucleotide reductase induced by herpes simplex type 1 virus. Characterization of a distinct enzyme. J Biol Chem. 1983;258:9831–9838. - PubMed
- Bianchi V, Pontis E, Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem. 1986;261:16037–16042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous