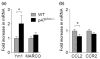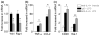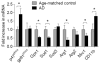Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation - PubMed (original) (raw)
Inhibition of NADPH oxidase promotes alternative and anti-inflammatory microglial activation during neuroinflammation
Sang-Ho Choi et al. J Neurochem. 2012 Jan.
Abstract
Like macrophages, microglia are functionally polarized into different phenotypic activation states, referred as classical and alternative. The balance of the two phenotypes may be critical to ensure proper brain homeostasis, and may be altered in brain pathological states, such as Alzheimer's disease. We investigated the role of NADPH oxidase in microglial activation state using p47(phox) and gp91(phox) -deficient mice as well as apocynin, a NADPH oxidase inhibitor during neuroinflammation induced by an intracerebroventricular injection of LPS or Aβ₁₋₄₂. We showed that NADPH oxidase plays a critical role in the modulation of microglial phenotype and subsequent inflammatory response. We demonstrated that inhibition of NADPH oxidase or gene deletion of its functional p47(phox) subunit switched microglial activation from a classical to an alternative state in response to an inflammatory challenge. Moreover, we showed a shift in redox state towards an oxidized milieu and that subpopulations of microglia retain their detrimental phenotype in Alzheimer's disease brains. Microglia can change their activation phenotype depending on NADPH oxidase-dependent redox state of microenvironment. Inhibition of NADPH oxidase represents a promising neuroprotective approach to reduce oxidative stress and modulate microglial phenotype towards an alternative state.
Published 2011. This article is a US Government work and is in the public domain in the USA.
Figures
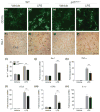
Fig. 1
p47phox−/− mice show reduced microglia accumulation. (a–h) Immunostaining for microglia in the hippocampus of p47phox−/− and WT mice at 24 h after LPS, with antibodies to CD11b and Iba-1. Scale bar: (a–d) 50 μm; (e–h) 100 μm. (i) Quantitation of Iba-1+ cells showed a significant decrease of microglia accumulation in p47phox−/− mice compared with WT mice. Mean ± SEM (n = 6), **p < 0.01. (j–n) Quantitative real-time PCR analysis showing expression of Iba-1, TNF-α, CCL2, CCR2, and IL-1β (relative to Pgk1) in p47phox−/− and WT mice. Mean ± SEM (n = 6), **p < 0.01.
Fig. 2
p47phox−/− mice show reduced peripheral leukocyte infiltration. (a–d) Immunostaining for neutrophils in the hippocampus of p47phox−/− and WT mice at 24 h after LPS, with 7/4 antibody. Scale bar, 100 μm. (e) Quantitation of 7/4+ cells showed a significant decrease of neutrophil infiltration in p47phox−/− mice compared with WT mice. Mean ± SEM (n = 6), *p < 0.05. (f) Brain MPO protein levels were measured in supernatants from WT and p47phox−/− mice at 24 h after LPS by ELISA. Mean ± SEM (n = 5), **p < 0.01.
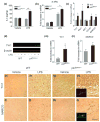
Fig. 3
Gene deletion of p47phox alters microglia phenotype. (a) ELISA showing production of brain IL-4 in p47phox−/− and WT mice. (b, c) Quantitative real-time PCR analysis showing expression of IL-4Rα, Ym1, Fizz1, Mrc1, CD163, and MARCO mRNA (relative to Pgk1) in p47phox−/− and WT mice. Mean ± SEM (n = 6), *p < 0.05, **p < 0.01. (d) Immunoblot showing protein levels of Ym1 in p47phox−/− and WT mice. (e–l) Immunostaining for the Ym1 and MARCO in the hippocampus of p47phox−/− and WT mice. Scale bar, 100 μm. Insets, immunofluorescence staining of Ym1+ and MARCO+ cells in p47phox−/− mice. (m, n) Induction of microglial Ym1 and MARCO. Brain microglia were isolated from WT and p47phox−/− mice at 24 h after LPS challenge and analyzed mRNA expression of Ym1 and MARCO by quantitative PCR. Mean ± SEM (n = 3), *p < 0.05. RNA, protein, and tissue samples were prepared from brains of WT and p47phox−/− mice at 24 h after LPS.
Fig. 4
Ym1 is primarily expressed in alternatively activated microglia during inflammatory response. (a–c) Immunofluorescence staining with antibodies to MAR-CO, GFAP, NeuN, and Ym1 in the hippocampus of p47phox−/− mice, showing that the Ym1+ cells overlaps precisely with MARCO+ microglia. Tissue samples were prepared from brains of p47phox−/− mice at 24 h after LPS. Scale bar, 25 μm.
Fig. 5
Inhibition of NADPH oxidase and gene deletion of gp91phox modulates microglial activation phenotypes. (a–d) Quantitative real-time PCR analysis showing expression of Ym1, MARCO, TNF-α, and CCL2 mRNA (relative to Pgk1) in WT mice pre-treated with apocynin. (e–h) The Ym1, MARCO, TNF-α, and CCR2 mRNA expression (relative to Pgk1) in WT and gp91phox−/− mice was measured by quantitative PCR. RNA samples were prepared from brains of WT and p47phox−/− mice at 24 h after LPS. Mean ± SEM (n = 5), *p < 0.05, **p < 0.01.
Fig. 6
Effects of p47phox deficiency on Aβ1–42-induced microglial activation. (a, b) Quantitative real-time PCR analysis showing expression of Ym1, MARCO, CCL2, and CCR2 mRNA (relative to Pgk1) in p47phox−/− and WT mice. RNA samples were prepared from brains of WT and p47phox−/− mice at 24 h after i.c.v. Aβ1–42. Mean ± SEM (n = 6), *p < 0.05
Fig. 7
Effects of IL-4 neutralization on LPS-induced microglial activation. (a–c) Quantitative real-time PCR analysis showing expression of Ym1, Fizz1, TNF-α, CCL2, CD40, and Iba-1 mRNA (relative to Pgk1) in IgG-treated or IL-4 neutralizing antibody-treated p47phox−/− mice. RNA samples were prepared from brains of WT and p47phox−/− mice at 24 h after coinjection of LPS and neutralizing antibody. Mean ± SEM (n = 6), *p < 0.05, **p < 0.01.
Fig. 8
Increased p47phox levels in the brain of AD patients. Quantitative PCR showing expression of p47phox and gp91phox, Gpx1, Sod1, and Arg1, Arg2, Mrc1, and CD11b mRNA in AD and age-matched controls by quantitative PCR. Mean ± SEM (n = 10), *p < 0.05.
Similar articles
- NADPH oxidases as potential pharmacological targets against increased seizure susceptibility after systemic inflammation.
Huang WY, Lin S, Chen HY, Chen YP, Chen TY, Hsu KS, Wu HM. Huang WY, et al. J Neuroinflammation. 2018 May 12;15(1):140. doi: 10.1186/s12974-018-1186-5. J Neuroinflammation. 2018. PMID: 29753328 Free PMC article. - Clozapine metabolites protect dopaminergic neurons through inhibition of microglial NADPH oxidase.
Jiang L, Wu X, Wang S, Chen SH, Zhou H, Wilson B, Jin CY, Lu RB, Xie K, Wang Q, Hong JS. Jiang L, et al. J Neuroinflammation. 2016 May 16;13(1):110. doi: 10.1186/s12974-016-0573-z. J Neuroinflammation. 2016. PMID: 27184631 Free PMC article. - Apocyanin, a Microglial NADPH Oxidase Inhibitor Prevents Dopaminergic Neuronal Degeneration in Lipopolysaccharide-Induced Parkinson's Disease Model.
Sharma N, Nehru B. Sharma N, et al. Mol Neurobiol. 2016 Jul;53(5):3326-3337. doi: 10.1007/s12035-015-9267-2. Epub 2015 Jun 17. Mol Neurobiol. 2016. PMID: 26081143 - Oxidative Stress, Neuroinflammation, and NADPH Oxidase: Implications in the Pathogenesis and Treatment of Alzheimer's Disease.
Ganguly U, Kaur U, Chakrabarti SS, Sharma P, Agrawal BK, Saso L, Chakrabarti S. Ganguly U, et al. Oxid Med Cell Longev. 2021 Apr 16;2021:7086512. doi: 10.1155/2021/7086512. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33953837 Free PMC article. Review. - NADPH oxidase as a therapeutic target in Alzheimer's disease.
Block ML. Block ML. BMC Neurosci. 2008 Dec 3;9 Suppl 2(Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. BMC Neurosci. 2008. PMID: 19090996 Free PMC article. Review.
Cited by
- The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases.
Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. Tan HY, et al. Oxid Med Cell Longev. 2016;2016:2795090. doi: 10.1155/2016/2795090. Epub 2016 Apr 6. Oxid Med Cell Longev. 2016. PMID: 27143992 Free PMC article. Review. - Two-dimensional zymography differentiates gelatinase isoforms in stimulated microglial cells and in brain tissues of acute brain injuries.
Chen S, Meng F, Chen Z, Tomlinson BN, Wesley JM, Sun GY, Whaley-Connell AT, Sowers JR, Cui J, Gu Z. Chen S, et al. PLoS One. 2015 Apr 10;10(4):e0123852. doi: 10.1371/journal.pone.0123852. eCollection 2015. PLoS One. 2015. PMID: 25859655 Free PMC article. - Central Nervous System Injury and Nicotinamide Adenine Dinucleotide Phosphate Oxidase: Oxidative Stress and Therapeutic Targets.
von Leden RE, Yauger YJ, Khayrullina G, Byrnes KR. von Leden RE, et al. J Neurotrauma. 2017 Feb 15;34(4):755-764. doi: 10.1089/neu.2016.4486. Epub 2016 Jun 27. J Neurotrauma. 2017. PMID: 27267366 Free PMC article. Review. - Microglia as Therapeutic Target for Radiation-Induced Brain Injury.
Liu Q, Huang Y, Duan M, Yang Q, Ren B, Tang F. Liu Q, et al. Int J Mol Sci. 2022 Jul 27;23(15):8286. doi: 10.3390/ijms23158286. Int J Mol Sci. 2022. PMID: 35955439 Free PMC article. Review. - Microglial response to aging and neuroinflammation in the development of neurodegenerative diseases.
Han T, Xu Y, Sun L, Hashimoto M, Wei J. Han T, et al. Neural Regen Res. 2024 Jun 1;19(6):1241-1248. doi: 10.4103/1673-5374.385845. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 37905870 Free PMC article.
References
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases