Composite effects of gene determinants on the translation speed and density of ribosomes - PubMed (original) (raw)
Composite effects of gene determinants on the translation speed and density of ribosomes
Tamir Tuller et al. Genome Biol. 2011.
Abstract
Background: Translation is a central process of life, and its regulation is crucial for cell growth. In this article, focusing on two model organisms, Escherichia coli and Saccharomyces cerevisiae, we study how three major local features of a gene's coding sequence (its adaptation to the tRNA pool, its amino acid charge, and its mRNA folding energy) affect its translation elongation.
Results: We find that each of these three different features has a non-negligible distinct correlation with the speed of translation elongation. In addition, each of these features might contribute independently to slowing down ribosomal speed at the beginning of genes, which was suggested in previous studies to improve ribosomal allocation and the cost of translation, and to decrease ribosomal jamming. Remarkably, a model of ribosomal translation based on these three basic features highly correlated with the genomic profile of ribosomal density. The robustness to transcription errors in terms of the values of these features is higher at the beginnings of genes, suggesting that this region is important for translation.
Conclusions: The reported results support the conjecture that translation elongation speed is affected by the three coding sequence determinants mentioned above, and not only by adaptation to the tRNA pool; thus, evolution shapes all these determinants along the coding sequences and across genes to improve the organism's translation efficiency.
Figures
Figure 1
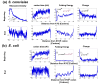
Mean genomic profiles of three features of the coding sequences. The mean genomic profiles of ribosome density, tAI, folding energy, and amino acid charge in S. cerevisiae and E. coli when aligning all the genes to their beginning (upper panels; Materials and methods) or end (lower panels; Materials and methods). In the case of the beginning profile, each panel also includes the region of the ramp (Materials and methods), a _P_-value corresponding to a comparison of the ramp to the rest of the profile (black; Materials and methods), and a _P_-value corresponding to a control for amino acid content (brown; Materials and methods).
Figure 2
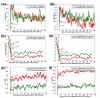
Profiles of charge, folding energy, and co-adaptation between codon bias and the tRNA pool for genes with high and low ribosomal density. (a-c) Profiles of charge (a), folding energy (b) and co-adaptation between codon bias and the tRNA pool (c) for genes with high ribosomal density (red; top 10%) and genes with low ribosomal density (green; bottom 10%) in S. cerevisiae. (d-f) Profiles of charge (d), folding energy (e) and co-adaptation between codon bias and the tRNA pool (f) for genes with high (mRNA levels) × (Ribosomal density) (red; top 10%) and genes with low (mRNA levels) × (ribosomal density) (green; bottom 10%) in S. cerevisiae. The ramps and corresponding _P_-values are shown in the figure. Similar graphs were obtained for E. coli and C. elegans (see graphs and corresponding _P_-values in Figures S4 and S5 in Additional file 2).
Figure 3
Genomic profiles of robustness to transcription error demonstrate that there is an increased selection for robustness at the beginning of genes in S. cerevisiae. (a) An illustration of the robustness computation: for each sliding window (length 13 codons) and in every coding sequence we computed the mean distance (in terms of folding energy (FE), tAI, and charge) from all its single nucleotide point mutations. In the next stage, the genomic profiles of robustness were plotted and analyzed (see more details in the Materials and methods). (b) The genomic profiles of robustness to transcription error (mean number of mutations that do not change the mRNA folding). The number of transcription errors (point mutations) that change the folding energy is lower at the beginning of genes (P = 1.4 × 10-100, Kolmogorov-Smirnov (KS) test). (c) The profiles of robustness to transcription error for five bins of equal size corresponding to the folding energy of the windows (Materials and methods; the boundaries of each bin are reported in the figures). The increased robustness at the beginning of genes remains significant even when controlling for local folding energy of the mRNA sequences. (d) The robustness to transcriptional errors in terms of folding energy is stronger than in randomized sequences (that maintain the codon bias and amino acid content of the original sequences; Materials and methods) at the beginning of genes (P = 6.5 × 10-5, KS test). (e, f) Profile of the robustness to transcriptional errors in terms of charge (e) and tAI (f). There is increased robustness at the beginning of genes in terms of the charge (e) as well as in terms of tAI (f). Ramp length and corresponding _P_-values are reported in the figures.
Figure 4
Predictions and modeling of the ribosomal density profile based on the features of the coding sequence. (a) Genomic profiles of tAI, folding, charge, a linear regressor based on all these variables, and ribosomal density. In the case of the linear regressor, the predicted ribosomal density is plotted as a function of the distance from the beginning of the ORF (x-axis). The linear regressor is a better predictor of the ribosomal density profile than each of these variables separately (dot plots in Figure S32 in Additional file 2). (b, c) Modeling of ribosomal velocity and density. (b) The velocity of translating the _i_-th codon is a function of the co-adaptation of the codon to the tRNA pool of the organism, the tAI, the folding energy (FE) after the codon (40 nucleotides), and the charge of the amino acids before the codon (31 amino acids; Materials and methods). (c) To compute the actual velocity we should also consider the initiation and termination times and the fact that a ribosome may be delayed by the ribosome in front of it due to 'traffic jams' (Materials and methods). (d) The profile of ribosomal density (red) versus the predicted profile of translation times based on a deterministic model (green; see (b, c)) and the predicted profile based on a stochastic model (blue; Materials and methods). (e) Correlations of various predictors of ribosomal density with the actual ribosomal density. The predictor that is based on the three variables and the model of ribosomal movement achieved the highest correlation.
Figure 5
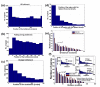
Relationship between ribosomal density, local tAI, folding, and charge in single genes of S. cerevisiae. (a) Histogram of the positions of the tAI bottleneck (the region with the slowest tAI). (b) Histogram of the positions of the folding energy bottleneck (the region with the strongest folding energy). (c) Histogram of the positions of the charge bottleneck (the region with the highest positive charge). (d) Histogram of the positions of the region with the highest ribosomal density. (e) Histogram of the distance between the composite bottleneck (based on the regressor that weighs the tAI, charge, and folding energy; Materials and methods) and the region with the highest ribosomal density positions. (f) Histogram of the distance between the composite bottleneck (based on the ribosome movement model; Materials and methods) and the region with the highest ribosomal density positions. Similar results were observed when we performed cross-validations.
Similar articles
- Determinants of translation elongation speed and ribosomal profiling biases in mouse embryonic stem cells.
Dana A, Tuller T. Dana A, et al. PLoS Comput Biol. 2012;8(11):e1002755. doi: 10.1371/journal.pcbi.1002755. Epub 2012 Nov 1. PLoS Comput Biol. 2012. PMID: 23133360 Free PMC article. - Mechanistic Modeling of In Vivo Translation in Escherichia coli Reliably Identifies Well-Adapted and Optimized RNA Sequences.
Spindler J, Giakissiklis C, Stierle C, Buschlüter M, Liebeton K, Siemann-Herzberg M, Takors R. Spindler J, et al. ACS Synth Biol. 2025 Mar 21;14(3):699-710. doi: 10.1021/acssynbio.4c00578. Epub 2025 Feb 27. ACS Synth Biol. 2025. PMID: 40014843 - Protein synthesis rates and ribosome occupancies reveal determinants of translation elongation rates.
Riba A, Di Nanni N, Mittal N, Arhné E, Schmidt A, Zavolan M. Riba A, et al. Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15023-15032. doi: 10.1073/pnas.1817299116. Epub 2019 Jul 10. Proc Natl Acad Sci U S A. 2019. PMID: 31292258 Free PMC article. - [tRNA-binding centers of Escherichia coli ribosomes and their structural organization].
Karpova GG. Karpova GG. Mol Biol (Mosk). 1984 Sep-Oct;18(5):1194-207. Mol Biol (Mosk). 1984. PMID: 6209546 Review. Russian. - tRNA engineering for manipulating genetic code.
Katoh T, Iwane Y, Suga H. Katoh T, et al. RNA Biol. 2018;15(4-5):453-460. doi: 10.1080/15476286.2017.1343227. Epub 2017 Sep 6. RNA Biol. 2018. PMID: 28722545 Free PMC article. Review.
Cited by
- Determinants of translation elongation speed and ribosomal profiling biases in mouse embryonic stem cells.
Dana A, Tuller T. Dana A, et al. PLoS Comput Biol. 2012;8(11):e1002755. doi: 10.1371/journal.pcbi.1002755. Epub 2012 Nov 1. PLoS Comput Biol. 2012. PMID: 23133360 Free PMC article. - Next-generation analysis of gene expression regulation--comparing the roles of synthesis and degradation.
McManus J, Cheng Z, Vogel C. McManus J, et al. Mol Biosyst. 2015 Oct;11(10):2680-9. doi: 10.1039/c5mb00310e. Mol Biosyst. 2015. PMID: 26259698 Free PMC article. Review. - A possible universal role for mRNA secondary structure in bacterial translation revealed using a synthetic operon.
Chemla Y, Peeri M, Heltberg ML, Eichler J, Jensen MH, Tuller T, Alfonta L. Chemla Y, et al. Nat Commun. 2020 Sep 24;11(1):4827. doi: 10.1038/s41467-020-18577-4. Nat Commun. 2020. PMID: 32973167 Free PMC article. - Parsing the synonymous mutations in the maize genome: isoaccepting mutations are more advantageous in regions with codon co-occurrence bias.
Chu D, Wei L. Chu D, et al. BMC Plant Biol. 2019 Oct 14;19(1):422. doi: 10.1186/s12870-019-2050-1. BMC Plant Biol. 2019. PMID: 31610786 Free PMC article. - Codon stabilization coefficient as a metric to gain insights into mRNA stability and codon bias and their relationships with translation.
Carneiro RL, Requião RD, Rossetto S, Domitrovic T, Palhano FL. Carneiro RL, et al. Nucleic Acids Res. 2019 Mar 18;47(5):2216-2228. doi: 10.1093/nar/gkz033. Nucleic Acids Res. 2019. PMID: 30698781 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases