A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer - PubMed (original) (raw)
. 2012 Jan 1;72(1):154-64.
doi: 10.1158/0008-5472.CAN-11-2484. Epub 2011 Nov 4.
Sippy Kaur, Joel Greshock, Heini Lassus, Xiaomin Zhong, Yanling Wang, Arto Leminen, Zhongjun Shao, Xiaowen Hu, Shun Liang, Dionyssios Katsaros, Qihong Huang, Ralf Bützow, Barbara L Weber, George Coukos, Lin Zhang
Affiliations
- PMID: 22058146
- PMCID: PMC4101815
- DOI: 10.1158/0008-5472.CAN-11-2484
A combined array-based comparative genomic hybridization and functional library screening approach identifies mir-30d as an oncomir in cancer
Ning Li et al. Cancer Res. 2012.
Abstract
Oncomirs are microRNAs (miRNA) that acts as oncogenes or tumor suppressor genes. Efficient identification of oncomirs remains a challenge. Here we report a novel, clinically guided genetic screening approach for the identification of oncomirs, identifying mir-30d through this strategy. mir-30d regulates tumor cell proliferation, apoptosis, senescence, and migration. The chromosomal locus harboring mir-30d was amplified in more than 30% of multiple types of human solid tumors (n = 1,283). Importantly, higher levels of mir-30d expression were associated significantly with poor clinical outcomes in ovarian cancer patients (n = 330, P = 0.0016). Mechanistic investigations suggested that mir-30d regulates a large number of cancer-associated genes, including the apoptotic caspase CASP3. The guided genetic screening approach validated by this study offers a powerful tool to identify oncomirs that may have utility as biomarkers or targets for drug development.
©2011 AACR.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
Figure 1
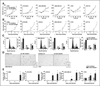
A clinically guided genetic screening approach to identify oncomirs A, aCGH frequency plots of ovarian (n = 109) and breast (n = 73) cancer specimens. Columns represent miRNAs which are ordered on the basis of location in the genome (from chromosome 1 to 22). B, Venn diagrams of miRNA genes with copy number gain shared by ovarian and breast cancers. C, a clinically guided genetic screening approach to identify oncomirs in vitro.
Figure 2
mir-30d regulates cancer cell proliferation and senescence in vitro. A, growth rates of the mir-30d mimic and control transfected tumor cells, measured using the MTT assay. B, growth rates of the mir-30d miRZips inhibitor and control miRZips expressed in tumor cells, measured by cell counts. C, cell-cycle analysis of the mir-30d miRZips inhibitor and control miRZips expressed in tumor cells using flow cytometry. D, β-Gal staining in control and mir-30d inhibitor transfected cells. E, percentage of β-Gal-positive cells after control and mir-30d inhibitor lentiviral transduction. OD, optical density. *P < 0.05.
Figure 3
mir-30d regulates cancer cell apoptosis in vitro. Tumor cells were transfected with mir-30d mimic or control mimic using Lipofectamine RNAiMAX transfection reagent. A, caspase-3 activity after control or camptothecin treatment, measured using the caspase-3 assay. B, total and cleaved caspase-3 after control and camptothecin treatment, detected by Western blots. C, percentage of apoptotic cells measured by flow cytometry using an Annexin V assay. D, total and cleaved PARP protein after control and camptothecin treatment, detected by Western blots. *P < 0.05.
Figure 4
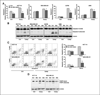
Inhibition of mir-30d blocks xenograft tumor growth in vivo. A and B, the control and mir-30d knockdown tumors (MDA-MB-231) were injected subcutaneously (without Matrigel) into female nude mice (n = 5). Tumor growth rates were measured and followed up for 38 days after tumor cell injection. C, the control and mir-30d knockdown tumors (MDA-MB-231) were injected subcutaneously (with Matrigel) into female nude mice (n = 5). Tumor growth rates were measured and followed up for 38 days after tumor cell injection. D, the xenograft tumors (with Matrigel) were monitored using a bioluminescence optical imaging system at day 38 postinjection. Right: control; left: mir-30d inhibitor. E, quantitative data of the optical imaging shown as photons (p) per second. *P < 0.05.
Figure 5
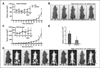
mir-30d genomic amplification and clinical significance in cancer patients. A, correlation analysis between mir-30d DNA copy number alteration and miRNA expression in cell lines. DNA copy number was analyzed by aCGH. Mature mir-30d expression was quantified by real-time RT-PCR. B, mir-30d exhibited genomic amplifications at high frequencies in multiple human solid tumors by aCGH (left) and SNP array (right). C, SNP array validation in human breast, colon, and lung cancer cell lines. D, mir-30d expression in ovarian cancer was detected by in situ hybridization. E, overall survival in patients with ovarian cancer (n = 330) according to mir-30d staining scores.
Figure 6
mir-30d regulates a large number of transcripts/genes in cancer cells. A and C, the genome-wide transcriptional effect of mir-30d mimic transfection compared with control mimic transfection was analyzed using an Affymetrix Human Gene 1.0 ST oligonucleotide array in ovarian cancer cell line 2008 (A) and breast cancer cell line MDA-MB-231 (C). Light gray spots indicate downregulated transcripts. B and D, histograms of negative natural logarithms of P values derived from a 1-tailed Wilcoxon rank sum test applied to the distributions of hexamers in the 3′-UTRs of all downregulated versus unchanged transcripts. E, Venn diagrams of transcript numbers shared by downregulated transcripts in mir-30d mimic transfections in 2008 and MDA-MB-231 cells and predicted targets of mir-30d by TargetScan. F, the microarray results were validated by real-time RT-PCR in 4 tumor cells. Real-time RT-PCR validation of transcripts that were downregulated in both 2008 and MDA-MB-231 cells after transfection with the mir-30d mimic and that were also predicted mir-30d targets by TargetScan (E). Validations were done in 2008 and MDA-MB-231 cells, as well as in 2 independent cell lines, HCT116and A2780. *, P < 0.05; **, P < 0.01.
Figure 7
mir-30d regulates CASP3 in cancer cells. A, the schematic diagram of the mir-30d binding site in the CASP3 3′-UTR, which was broadly conserved among different species. Hsa, Human; Ptr, chimpanzee; Mml, Rhesus; Mmu, mouse; Rno, Rat; Cpo, Pig; Sar, Shrew; Cfa, Dog; Fca: Cat; Eca, Horse; Bta, Cow; Dno, armadillo; Laf, elephant. B, the mir-30d mimics and control mimic were transiently transfected into 4 cancer cell lines. At 48 hours posttransfection, total RNA were collected, and the endogenous CASP3 expression was examined by real-time RT-PCR. C, reporter assays using wild type and mir-30d binding site mutant CASP-3 3′-UTR. The mir-30d mimic transfection significantly reduced luciferase activity in the wild type but not the binding site mutant CASP3 3′-UTR reporters. WT, wild type. *P < 0.05; **P < 0.01.
Similar articles
- mir-30d Regulates multiple genes in the autophagy pathway and impairs autophagy process in human cancer cells.
Yang X, Zhong X, Tanyi JL, Shen J, Xu C, Gao P, Zheng TM, DeMichele A, Zhang L. Yang X, et al. Biochem Biophys Res Commun. 2013 Feb 15;431(3):617-22. doi: 10.1016/j.bbrc.2012.12.083. Epub 2012 Dec 27. Biochem Biophys Res Commun. 2013. PMID: 23274497 Free PMC article. - MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features.
Lee H, Park CS, Deftereos G, Morihara J, Stern JE, Hawes SE, Swisher E, Kiviat NB, Feng Q. Lee H, et al. World J Surg Oncol. 2012 Aug 27;10:174. doi: 10.1186/1477-7819-10-174. World J Surg Oncol. 2012. PMID: 22925189 Free PMC article. - MicroRNA-30d inhibits the migration and invasion of human esophageal squamous cell carcinoma cells via the post‑transcriptional regulation of enhancer of zeste homolog 2.
Xie R, Wu SN, Gao CC, Yang XZ, Wang HG, Zhang JL, Yan W, Ma TH. Xie R, et al. Oncol Rep. 2017 Mar;37(3):1682-1690. doi: 10.3892/or.2017.5405. Epub 2017 Jan 25. Oncol Rep. 2017. PMID: 28184915 - Epithelial-mesenchymal transition-associated miRNAs in ovarian carcinoma, with highlight on the miR-200 family: prognostic value and prospective role in ovarian cancer therapeutics.
Koutsaki M, Spandidos DA, Zaravinos A. Koutsaki M, et al. Cancer Lett. 2014 Sep 1;351(2):173-81. doi: 10.1016/j.canlet.2014.05.022. Epub 2014 Jun 18. Cancer Lett. 2014. PMID: 24952258 Review. - OncomiRs: the discovery and progress of microRNAs in cancers.
Cho WC. Cho WC. Mol Cancer. 2007 Sep 25;6:60. doi: 10.1186/1476-4598-6-60. Mol Cancer. 2007. PMID: 17894887 Free PMC article. Review.
Cited by
- Prognostic and Predictive Epigenetic Biomarkers in Oncology.
Kamińska K, Nalejska E, Kubiak M, Wojtysiak J, Żołna Ł, Kowalewski J, Lewandowska MA. Kamińska K, et al. Mol Diagn Ther. 2019 Feb;23(1):83-95. doi: 10.1007/s40291-018-0371-7. Mol Diagn Ther. 2019. PMID: 30523565 Free PMC article. Review. - Role of microRNA-30c targeting ADAM19 in colorectal cancer.
Zhang Q, Yu L, Qin D, Huang R, Jiang X, Zou C, Tang Q, Chen Y, Wang G, Wang X, Gao X. Zhang Q, et al. PLoS One. 2015 Mar 23;10(3):e0120698. doi: 10.1371/journal.pone.0120698. eCollection 2015. PLoS One. 2015. PMID: 25799050 Free PMC article. - Confirmation of a metastasis-specific microRNA signature in primary colon cancer.
Coebergh van den Braak RRJ, Sieuwerts AM, Lalmahomed ZS, Smid M, Wilting SM, Bril SI, Xiang S, van der Vlugt-Daane M, de Weerd V, van Galen A, Biermann K, van Krieken JHJM, Kloosterman WP, Foekens JA; MATCH study group*; Martens JWM, IJzermans JNM. Coebergh van den Braak RRJ, et al. Sci Rep. 2018 Mar 27;8(1):5242. doi: 10.1038/s41598-018-22532-1. Sci Rep. 2018. PMID: 29588449 Free PMC article. - A serum microRNA signature as a prognostic factor for patients with advanced NSCLC and its association with tissue microRNA expression profiles.
Guo J, Meng R, Yin Z, Li P, Zhou R, Zhang S, Dong X, Liu L, Wu G. Guo J, et al. Mol Med Rep. 2016 Jun;13(6):4643-53. doi: 10.3892/mmr.2016.5114. Epub 2016 Apr 13. Mol Med Rep. 2016. PMID: 27081922 Free PMC article. - MicroRNA-1908 functions as a glioblastoma oncogene by suppressing PTEN tumor suppressor pathway.
Xia X, Li Y, Wang W, Tang F, Tan J, Sun L, Li Q, Sun L, Tang B, He S. Xia X, et al. Mol Cancer. 2015 Aug 12;14:154. doi: 10.1186/s12943-015-0423-0. Mol Cancer. 2015. PMID: 26265437 Free PMC article.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
- McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13:253–258. - PubMed
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. - PubMed
- Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. - PubMed
- Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials