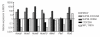Functional analysis of the NUP98-CCDC28A fusion protein - PubMed (original) (raw)
doi: 10.3324/haematol.2011.047969. Epub 2011 Nov 4.
Christine Ragu, Gwendoline Soler, Chris Ottolenghi, Caroline Schluth, Isabelle Radford-Weiss, Sylvie Schneider-Maunoury, Isabelle Callebaut, Nicole Dastugue, Harry A Drabkin, Olivier A Bernard, Serge Romana, Virginie Penard-Lacronique
Affiliations
- PMID: 22058212
- PMCID: PMC3291592
- DOI: 10.3324/haematol.2011.047969
Functional analysis of the NUP98-CCDC28A fusion protein
Arnaud Petit et al. Haematologica. 2012 Mar.
Abstract
Background: The nucleoporin gene NUP98 is rearranged in more than 27 chromosomal abnormalities observed in childhood and adult, de novo and therapy-related acute leukemias of myeloid and T-lymphoid origins, resulting in the creation of fusion genes and the expression of chimeric proteins. We report here the functional analysis of the NUP98-coiled-coil domain-containing protein 28A (NUP98-CCDC28A) fusion protein, expressed as the consequence of a recurrent t(6;11)(q24.1;p15.5) translocation.
Design and methods: To gain insight into the function of the native CCDC28A gene, we collected information on any differential expression of CCDC28A among normal hematologic cell types and within subgroups of acute leukemia. To assess the in vivo effects of the NUP98-CCDC28A fusion, NUP98-CCDC28A or full length CCDC28A were retrovirally transduced into primary murine bone marrow cells and transduced cells were next transplanted into sub-lethally irradiated recipient mice.
Results: Our in silico analyses supported a contribution of CCDC28A to discrete stages of murine hematopoietic development. They also suggested selective enrichment of CCDC28A in the French-American-British M6 class of human acute leukemia. Primary murine hematopoietic progenitor cells transduced with NUP98-CCDC28A generated a fully penetrant and transplantable myeloproliferative neoplasm-like myeloid leukemia and induced selective expansion of granulocyte/macrophage progenitors in the bone marrow of transplanted recipients, showing that NUP98-CCDC28A promotes the proliferative capacity and self-renewal potential of myeloid progenitors. In addition, the transformation mediated by NUP98-CCDC28A was not associated with deregulation of the Hoxa-Meis1 pathway, a feature shared by a diverse set of NUP98 fusions.
Conclusions: Our results demonstrate that the recurrent NUP98-CCDC28A is an oncogene that induces a rapid and transplantable myeloid neoplasm in recipient mice. They also provide additional evidence for an alternative leukemogenic mechanism for NUP98 oncogenes.
Figures
Figure 1
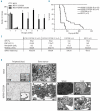
The t(6;11)(q24.1;p15.5) translocation fuses NUP98 to CCDC28A and leads to the expression of a NUP98-CCDC28A protein localized to the nucleus. (A) Nucleotide and amino acid sequences around the NUP98-CCDC28A fusion junction. A specific PCR product of 503 bp was obtained using the DNA from the patient’s sample (P) but not from control genomic DNA (C) (panel a); the fusion joins the nucleotide (Nt) 62503 of NUP98 to the Nt 653 of CCDC28A. RT-PCR experiments performed on RNA extracted from the leukemic sample (P) show the amplification of two specific 444 bp and 612 bp products, corresponding respectively to the NUP98-CCDC28A (panel b) and reciprocal CCDC28A-NUP98 (panel c) fusion transcripts. The nucleotide sequence of the NUP98-CCDC28A transcript shows an in-frame fusion, joining the Nt 1833 of NUP98 to the Nt 384 of CCDC28A. The reciprocal CCDC28A-NUP98 transcript joins the Nt 383 of CCDC28A to the Nt 2022 of NUP98 but harbors a non-sense codon. M, molecular weight markers. (B) Schematic representation of the NUP98, CCDC28A and NUP98-CCDC28A human proteins. Identified domains [GLFG-repeats; RNA binding domain (RBD)] and the predicted coiled-coil domain (CCD) are indicated. The chimeric exon-exon boundary joins NUP98 to amino acid position 77 of the putative L-isoform, leading to a 712 amino acid-long fusion protein. (C) Clustal-W alignment of the predicted proteins for mouse CCDC28A (NP_659069), human CCDC28A (NP_056254) and for human CCDC28B (NP_077272). The coding potential of the mouse cDNA for CCDC28A is extended N-terminal of the first methionine, in order to show partial alignment for a short segment along with three in-frame stop codons (*). Human CCDC28A is labeled with an “-L” suffix to indicate the putative long isoform (see text). The symbols placed below the alignment are as follows: “!”, first amino acid of the sequence from CCDC28A that is joined to NUP98 in leukemia; “#”, first methionine for the “S-isoform” of human CCDC28A and for the two other proteins; “+”, amino acid positions that are fully conserved in the three proteins; “:” and “.”, amino acid positions with decreasing degrees of partial conservation. Above the alignment, the “%” symbols indicate exon-exon junctions for CCDC28A. In the murine CCDC28A protein, a short orthologous segment can be recognized upstream of the conserved methionine present in the human protein but contains in-frame stop codons. (D) Immunocytochemistry with an anti-hemagglutinin antibody detecting the nuclei of murine fibroblasts transiently transfected with constructs encoding the hemagglutinin-tagged NUP98-CCDC28A fusion protein (panel a) and S-isoform of CCDC28A (panel b); panel c is the negative control.
Figure 2
NUP98-CCDC28A expression induces a myeloproliferative neoplasm-like myeloid leukemia. (A) Clonogenic progenitor assays. Expression of NUP98-CCDC28A in primary bone marrow progenitors resulted in their enhanced proliferation in vitro. Colonies were scored every week and replated in secondary cultures. The mean number of colonies per round of replating of three independent replicates is indicated. The error bars indicate standard deviation (SD). (B) Kaplan-Meier survival curve of mice transplanted with bone marrow progenitors transduced with NUP98-CCDC28A (n=20), CCDC28A (n=5) or the vector alone (n=3). Primary NUP98-CCDC28A recipients (indicated as NUP98-CCDC28A IR) showed a 100% death rate at day 236 (7.8 months). Animals transduced with the CCDC28A or the MSCV vector alone were sacrificed for end-point analysis without evidence of disease. NUP98-CCDC28A secondary recipients (NUP98-CCDC28A IIR, n=8) succumbed between days 38 and 87 post-transplant. (C) Blood counts of primary _NUP98-CCDC28A_-transduced recipients (NUP98-CCDC28A IR) showed hyperleukocytosis, severe anemia and thrombocytopenia. These abnormalities were also observed in second recipients (NUP98-CCDC28A IIR) while _CCDC28A_- and MSCV-transduced mice showed normal blood count parameters. Spleen weights from primary transplanted mice are indicated. Values shown are mean ± SD. (D) Blood cytology and tissue histology of representative NUP98-CCDC28A and MSCV mice. Peripheral blood smears show anemia, thrombocytopenia and hyperleukocytosis for primary NUP98-CCDC28A animals (panel b) when compared to MSCV animals (panel a). In the former, maturation of myeloid forms to segmented neutrophils was observed (May-Grünwald-Giemsa staning, x100). Cytological analysis of bone marrow cells, evaluated on May-Grünwald-Giemsa staining of cytospin preparations, shows an over-representation of mature myeloid cells in _NUP98-CCDC28A_-engrafted mice (panel d) when compared to control animals (panel c) (May-Grünwald-Giemsa staining, x40). Histological analysis of the spleen (panel e), liver (panel f), kidney (panel g) and lung (panel h) of primary mice transplanted with bone marrow progenitors transduced with NUP98-CCDC28A, shows accumulation of myeloid precursors and destruction of normal organ architecture (hematoxylin and eosin, x20 and x200).
Figure 3
Leukemic cells from NUP98-CCDC28A mice are enriched in granulocytic-monocytic progenitors. Representative FACS profile of immature progenitors immunophenically defined as LSK (Lin-Sca1+c-Kit+) and myeloid progenitors (MP, Lin-Sca1−c-Kit+) in the bone marrow of _NUP98-CCDC28A_-engrafted mice. FACS analysis of Lin- cells shows the distribution of MP in the bone marrow of leukemic animals and specific expansion of a population immunophenotypically defined as granulocytic-monocytic progenitors (GMP), at the expense of the common myeloid progenitors (CMP) and megakaryocyte-erythroid progenitors (MEP) populations. A profile of bone marrow cells from mice transplanted with control-transduced progenitors shows typical GMP, CMP and MEP populations. A major reduction in LSK cells is observed in the bone marrow of _NUP98-CCDC28A_-engrafted mice. Histograms show the percentages of indicated cells in the bone marrow from leukemic and control mice (right panels). Values shown are mean ± standard error of the mean (SEM) (n=5 mice per group, Mann Whitney test).
Figure 4
NUP98-CCDC28A leukemic cells do not over-express HoxA genes. Real-time reverse transcriptase-PCR analysis of transcript levels of HoxA5, HoxA7, HoxA9, HoxA10, Meis1, Pbx1 and Pbx3 genes in the bone marrow cells from primary NUP98-CCDC28A-engrafted animals and their CCDC28A- and MSCV-transduced conterparts. Accumulation of transcript is quantified in primary recipients compared to NUP98-HOXA9 and MPL T487A recipients, respectively used as positive and negative controls of dysregulated expression of HoxA genes. Expression levels are normalized to Gapdh and results are expressed relative to the level of each gene in MSCV-engrafted mice (set at 1) (n=3 per genotype). Values shown are mean ± SD from two independent experiments.
Similar articles
- Induction of acute myeloid leukemia in mice by the human leukemia-specific fusion gene NUP98-HOXD13 in concert with Meis1.
Pineault N, Buske C, Feuring-Buske M, Abramovich C, Rosten P, Hogge DE, Aplan PD, Humphries RK. Pineault N, et al. Blood. 2003 Jun 1;101(11):4529-38. doi: 10.1182/blood-2002-08-2484. Epub 2003 Jan 23. Blood. 2003. PMID: 12543865 - Differential and common leukemogenic potentials of multiple NUP98-Hox fusion proteins alone or with Meis1.
Pineault N, Abramovich C, Ohta H, Humphries RK. Pineault N, et al. Mol Cell Biol. 2004 Mar;24(5):1907-17. doi: 10.1128/MCB.24.5.1907-1917.2004. Mol Cell Biol. 2004. PMID: 14966272 Free PMC article. - NUP98-HOXA9 bearing therapy-related myeloid neoplasm involves myeloid-committed cell and induces HOXA5, EVI1, FLT3, and MEIS1 expression.
Burillo-Sanz S, Morales-Camacho RM, Caballero-Velázquez T, Vargas MT, García-Lozano JR, Falantes JF, Prats-Martín C, Bernal R, Pérez-Simón JA. Burillo-Sanz S, et al. Int J Lab Hematol. 2016 Feb;38(1):64-71. doi: 10.1111/ijlh.12435. Epub 2015 Sep 29. Int J Lab Hematol. 2016. PMID: 26418229 - Fusion of the NUP98 gene and the homeobox gene HOXC13 in acute myeloid leukemia with t(11;12)(p15;q13).
Panagopoulos I, Isaksson M, Billström R, Strömbeck B, Mitelman F, Johansson B. Panagopoulos I, et al. Genes Chromosomes Cancer. 2003 Jan;36(1):107-12. doi: 10.1002/gcc.10139. Genes Chromosomes Cancer. 2003. PMID: 12461755 Review. - NUP98 fusion in human leukemia: dysregulation of the nuclear pore and homeodomain proteins.
Nakamura T. Nakamura T. Int J Hematol. 2005 Jul;82(1):21-7. doi: 10.1532/IJH97.04160. Int J Hematol. 2005. PMID: 16105755 Review.
Cited by
- Nucleoporin genes in human diseases.
Nofrini V, Di Giacomo D, Mecucci C. Nofrini V, et al. Eur J Hum Genet. 2016 Oct;24(10):1388-95. doi: 10.1038/ejhg.2016.25. Epub 2016 Apr 13. Eur J Hum Genet. 2016. PMID: 27071718 Free PMC article. Review. - Mechanistic insights and potential therapeutic approaches for NUP98-rearranged hematologic malignancies.
Michmerhuizen NL, Klco JM, Mullighan CG. Michmerhuizen NL, et al. Blood. 2020 Nov 12;136(20):2275-2289. doi: 10.1182/blood.2020007093. Blood. 2020. PMID: 32766874 Free PMC article. Review. - Identification and validation of a seven-gene prognostic marker in colon cancer based on single-cell transcriptome analysis.
Zhou Y, Guo Y, Wang Y. Zhou Y, et al. IET Syst Biol. 2022 Apr;16(2):72-83. doi: 10.1049/syb2.12041. IET Syst Biol. 2022. PMID: 35352485 Free PMC article. - Data on affected cancer-related genes in pediatric t(12;21)-positive acute lymphoblastic leukemia patients harboring unbalanced der(6)t(X;6) translocations.
Kjeldsen E. Kjeldsen E. Data Brief. 2016 Jul 5;8:894-903. doi: 10.1016/j.dib.2016.06.060. eCollection 2016 Sep. Data Brief. 2016. PMID: 27508240 Free PMC article. - CCDC28A deficiency causes sperm head defects, reduced sperm motility and male infertility in mice.
Zhou H, Zhang Z, Qu R, Zhu H, Luo Y, Li Q, Mu J, Yu R, Zeng Y, Chen B, Sang Q, Wang L. Zhou H, et al. Cell Mol Life Sci. 2024 Apr 10;81(1):174. doi: 10.1007/s00018-024-05184-5. Cell Mol Life Sci. 2024. PMID: 38597936 Free PMC article.
References
- Jeganathan KB, Baker DJ, van Deursen JM. Securin associates with APCCdh1 in prometaphase but its destruction is delayed by Rae1 and Nup98 until the meta-phase/anaphase transition. Cell Cycle. 2006;5(4):366–70. - PubMed
- Kalverda B, Pickersgill H, Shloma VV, Fornerod M. Nucleoporins directly stimulate expression of developmental and cell-cycle genes inside the nucleoplasm. Cell. 2010;140(3):360–71. - PubMed
- Romana SP, Radford-Weiss I, Ben Abdelali R, Schluth C, Petit A, Dastugue N, et al. NUP98 rearrangements in hematopoietic malignancies: a study of the Groupe Francophone de Cytogenetique Hematologique. Leukemia. 2006;20(4):696–706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases