Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection - PubMed (original) (raw)
Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection
John R Teijaro et al. J Immunol. 2011.
Abstract
We identify in this article a new class of lung tissue-resident memory CD4 T cells that exhibit tissue tropism and retention independent of Ag or inflammation. Tissue-resident memory CD4 T cells in the lung did not circulate or emigrate from the lung in parabiosis experiments, were protected from in vivo Ab labeling, and expressed elevated levels of CD69 and CD11a compared with those of circulating memory populations. Importantly, influenza-specific lung-resident memory CD4 T cells served as in situ protectors to respiratory viral challenge, mediating enhanced viral clearance and survival to lethal influenza infection. By contrast, memory CD4 T cells isolated from spleen recirculated among multiple tissues without retention and failed to mediate protection to influenza infection, despite their ability to expand and migrate to the lung. Our results reveal tissue compartmentalization as a major determining factor for immune-mediated protection in a key mucosal site, important for targeting local protective responses in vaccines and immunotherapies.
Figures
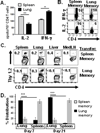
Figure 1. Lung memory CD4 T cells exhibit distinct migration tropism compared to spleen memory CD4 T cells
(A) Influenza-specific IL-2 and IFN-γ production by polyclonal influenza-specific lung and spleen memory CD4 T cells determined by ELISPOT. Results are expressed as mean spots +/− SD from triplicate wells, representative of 3 experiments. (B) Cytokine production by HA-specific memory CD4 T cells isolated from spleen or lungs of RAG2−/− adoptive hosts (see methods) following stimulation with HA peptide and APC. Intracellular cytokine staining is shown gated on CD4+6.5+ cells, representative of 3 experiments with 4 mice/group. (C) In vivo migration of lung and spleen HA-specific memory CD4 T cells into specific tissues 7 days after transfer into BALB/c(Thy 1.1) recipients. Plots show frequency of CD4+Thy1.2+ HA-specific memory CD4 T-cells in each site, representative of 3 experiments (5–9 mice/group). (D) Percent distribution of HA-specific memory CD4 T cells recovered from each tissue (7 and 21 days post-transfer) calculated as follows:% distribution=# Thy 1.2+memory CD4 T cells in a specific tissue# Thy 1.2+memory CD4 T cells from all tissues×100Histograms represent averages±s.d. from three independent experiments (5–7 mice/group). Significance indicated as *** for p <0.005.
Figure 2. Irreversible tissue retention of lung memory CD4 T cells
Host mice containing Thy1.1+ lung or spleen-derived HA-specific memory CD4 T cells as in Fig. 1C were surgically conjoined to syngeneic BALB/c partner mice in parabiosis experiments (see methods). (A) Frequency of spleen and lung-derived CD4+Thy1.1+ HA-specific memory CD4 T-cells (of total CD4 T cells) in tissues of host and parabiont partner mice after 8 days conjoined. (B) Percent distribution of HA-specific memory CD4 T cells recovered from host and partner mouse tissues calculated as in Fig. 1D. Results are compiled from eight parabiotic mouse pairs/group and representative of 2 independent experiments. Significant differences between lung and spleen memory migration are indicated by *** (p <0.005).
Figure 3. Distinct phenotype of tissue-resident versus circulating memory CD4 T cells
(A) Cell surface CD11a and CD69 expression by lung- and spleen-derived HA-specific memory CD4 T cells gated on live CD4+CD44hiCD62Llo T cells, and are representative of 4 independent experiments. (B) In vivo labeling delineates resident and circulating polyclonal lung memory CD4 T cell subsets. BALB/c mice previously infected with influenza (4–6 weeks post-infection) were injected intravenously with fluorescently labeled anti-CD4 antibody, and blood and lung tissue were harvested. Upper: Proportion of CD3ε+CD8α− γδ− T cells stained by or protected from in vivo administered antibody. Lower: CD11a and CD69 expression by the labeled or protected cell populations, representative of 4 independent experiments.
Figure 4. Lung memory CD4 T cells mediate enhanced protection to influenza challenge compared to spleen memory CD4 T cells
BALB/c mice and recipients of lung- or spleen-derived HA-specific memory CD4 T cells (106/mouse) were challenged with PR8 influenza virus. (A) Daily weight loss following sub-lethal influenza infection, compiled from 6 independent experiments with n=20–25 per group. (B) Kinetic analysis of influenza viral titers in the lungs of naive mice or mice receiving spleen- or lung-derived memory CD4 T cells as in A. Results are expressed as mean TCID50 from 4–9 mice per group, representative of 2–4 experiments at each time-point. (C) Lung memory CD4 T cells protect from lethal challenge. Graph shows survival of naive BALB/c mice or mouse recipients of spleen- or lung-derived memory CD4 T cells infected with 2LD50 of PR8 influenza virus from two independent experiments with 8–10 mice/group. (D) Numbers of lung- and spleen-derived HA-specific memory CD4 T cells recovered from recipient spleen and lungs 6 days post-infection, expressed as the average from 5 mice/group, representative of six experiments. Significance for all sections indicated by *** for p <0.0005, ** for p < 0.005, * for p < 0.05, and #, for p = 0.06.
Similar articles
- The Role of CD4+ Resident Memory T Cells in Local Immunity in the Mucosal Tissue - Protection Versus Pathology.
Hirahara K, Kokubo K, Aoki A, Kiuchi M, Nakayama T. Hirahara K, et al. Front Immunol. 2021 Apr 21;12:616309. doi: 10.3389/fimmu.2021.616309. eCollection 2021. Front Immunol. 2021. PMID: 33968018 Free PMC article. Review. - Lung niches for the generation and maintenance of tissue-resident memory T cells.
Turner DL, Bickham KL, Thome JJ, Kim CY, D'Ovidio F, Wherry EJ, Farber DL. Turner DL, et al. Mucosal Immunol. 2014 May;7(3):501-10. doi: 10.1038/mi.2013.67. Epub 2013 Sep 25. Mucosal Immunol. 2014. PMID: 24064670 Free PMC article. - Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1.
Chapman TJ, Topham DJ. Chapman TJ, et al. J Immunol. 2010 Apr 1;184(7):3841-9. doi: 10.4049/jimmunol.0902281. Epub 2010 Mar 3. J Immunol. 2010. PMID: 20200271 Free PMC article. - Memory CD4 T cell-mediated immunity against influenza A virus: more than a little helpful.
McKinstry KK, Dutton RW, Swain SL, Strutt TM. McKinstry KK, et al. Arch Immunol Ther Exp (Warsz). 2013 Oct;61(5):341-53. doi: 10.1007/s00005-013-0236-z. Epub 2013 May 25. Arch Immunol Ther Exp (Warsz). 2013. PMID: 23708562 Free PMC article. Review.
Cited by
- Transcriptional control of effector and memory CD8+ T cell differentiation.
Kaech SM, Cui W. Kaech SM, et al. Nat Rev Immunol. 2012 Nov;12(11):749-61. doi: 10.1038/nri3307. Epub 2012 Oct 19. Nat Rev Immunol. 2012. PMID: 23080391 Free PMC article. Review. - The Role of CD4+ Resident Memory T Cells in Local Immunity in the Mucosal Tissue - Protection Versus Pathology.
Hirahara K, Kokubo K, Aoki A, Kiuchi M, Nakayama T. Hirahara K, et al. Front Immunol. 2021 Apr 21;12:616309. doi: 10.3389/fimmu.2021.616309. eCollection 2021. Front Immunol. 2021. PMID: 33968018 Free PMC article. Review. - Heterosubtypic T-Cell Immunity to Influenza in Humans: Challenges for Universal T-Cell Influenza Vaccines.
Sridhar S. Sridhar S. Front Immunol. 2016 May 19;7:195. doi: 10.3389/fimmu.2016.00195. eCollection 2016. Front Immunol. 2016. PMID: 27242800 Free PMC article. Review. - Resident memory T cells form during persistent antigen exposure leading to allograft rejection.
Abou-Daya KI, Tieu R, Zhao D, Rammal R, Sacirbegovic F, Williams AL, Shlomchik WD, Oberbarnscheidt MH, Lakkis FG. Abou-Daya KI, et al. Sci Immunol. 2021 Mar 19;6(57):eabc8122. doi: 10.1126/sciimmunol.abc8122. Sci Immunol. 2021. PMID: 33741656 Free PMC article. - Higher frequency of peripheral blood CD103+CD8+ T cells with lower levels of PD-1 and TIGIT expression related to favorable outcomes in leukemia patients.
Liu L, Lai W, Zhuo X, Chen S, Luo X, Tan H. Liu L, et al. Front Immunol. 2024 Sep 25;15:1437726. doi: 10.3389/fimmu.2024.1437726. eCollection 2024. Front Immunol. 2024. PMID: 39391310 Free PMC article.
References
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. - PubMed
- Bingaman AW, Patke DS, Mane VR, Ahmadzadeh M, Ndejembi M, Bartlett ST, Farber DL. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur J Immunol. 2005;35:3173–3186. - PubMed
Publication types
MeSH terms
Grants and funding
- U19 AI083022/AI/NIAID NIH HHS/United States
- AI083022/AI/NIAID NIH HHS/United States
- AI76457/AI/NIAID NIH HHS/United States
- AI41576/AI/NIAID NIH HHS/United States
- R01 AI076457/AI/NIAID NIH HHS/United States
- R01 AI041576/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials