Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution - PubMed (original) (raw)
. 2012 Feb 28;158(1):148-55.
doi: 10.1016/j.jconrel.2011.10.021. Epub 2011 Oct 26.
Pilhan Kim, O'Hara Haley, Jean R Fakhoury, Giulia Adriani, Jeffrey Schmulen, Padraig Moloney, Fazle Hussain, Mauro Ferrari, Xuewu Liu, Seok-Hyun Yun, Paolo Decuzzi
Affiliations
- PMID: 22062689
- PMCID: PMC3422657
- DOI: 10.1016/j.jconrel.2011.10.021
Rapid tumoritropic accumulation of systemically injected plateloid particles and their biodistribution
Anne L van de Ven et al. J Control Release. 2012.
Abstract
Nanoparticles for cancer therapy and imaging are designed to accumulate in the diseased tissue by exploiting the Enhanced Permeability and Retention (EPR) effect. This limits their size to about 100nm. Here, using intravital microscopy and elemental analysis, we compare the in vivo localization of particles with different geometries and demonstrate that plateloid particles preferentially accumulate within the tumor vasculature at unprecedented levels, independent of the EPR effect. In melanoma-bearing mice, 1000×400nm plateloid particles adhered to the tumor vasculature at about 5% and 10% of the injected dose per gram organ (ID/g) for untargeted and RGD-targeted particles respectively, and exhibited the highest tumor-to-liver accumulation ratios (0.22 and 0.35). Smaller and larger plateloid particles, as well as cylindroid particles, were more extensively sequestered by the liver, spleen, and lungs. Plateloid particles appeared well-suited for taking advantage of hydrodynamic forces and interfacial interactions required for efficient tumoritropic accumulation, even without using specific targeting ligands.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
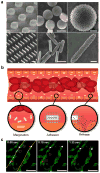
Figure 1. Rationally designed nanoporous silicon particles
a, SEM images of nanoporous plateloid (1000×400 nm) and cylindroid (1500×200 nm) silicon particles micropatterned from a porous film before (left) and after release (middle, right) (scale bars, 1.0, 1.0, and 0.25 μm). b, Longitudinal cross-section illustration of a blood vessel containing circulating red blood cells and silicon particles traveling through and adhering to the walls. Margination inset: The lateral drifting of anisometric particles promotes periodic interaction with the vessel walls; Adhesion inset: The large surface area of anisometric particles favors specific and non-specific interactions with receptors on the endothelial surface; Release inset: The release of cargo by adherent particles facilitates localized delivery into the underlying tissue. c, Intravital microscopy images showing time-dependent trajectories of individual 600×200 nm (red) and 1000×400 nm (blue) plateloid silicon particles in the ear venule of a Tie-2 GFP+ mouse. The triangles indicate relatively slow moving particles (μm/sec), the circles indicate fast moving particles (μm/msec), and the square indicates particles adhered to the vessel wall. Endothelial cells are colored green, vessel walls are demarcated in yellow (scale bar, 50 μm).
Figure 2. Biodistribution of plateloid particles in live mice
a, Distribution of 600×200 nm (red) and 1000×400 nm (blue) plateloid silicon particles in different organs of Tie2-GFP+ mice 1h after simultaneous systemic injection (scale bar, 50 μm). b, Time-dependent accumulation of plateloid particles (injected independently) in two major RES organs – the liver and the spleen – and in the tumor tissue of melanoma-bearing mice, measured using intravital microscopy. Data is plotted as a mean curve ± 1 SD (n=4 animals).
Figure 3. Cumulative particle localization at the organ level: The effect of particle geometry
a, Intravital data on the cumulative uptake of plateloid (top) and cylindroid (bottom) particles 30 minutes after injection. b, ICP-AES data on the cumulative uptake of plateloid (top) and cylindroid (bottom) particles expressed as a percentage of the injected dose normalized by the tissue mass. Similar trends are observed with the two independent techniques.
Figure 4. Accumulation of untargeted and RGD-targeted plateloid particles in melanoma tumors
a, Intravital microscopy data on the time-dependent accumulation of 600×200 nm (left), 1000×400 nm (center), and 1800×600 nm (right) plateloid particles. b, ICP-AES data on the cumulative particle uptake expressed as percentage of the injected dose normalized by the tumor mass. c, Stills extracted from intravital videos reveal that 1000×400 nm particles accumulate within the tumor vasculature (top left), whereas some 600×200 nm particles extravasate out of the vasculature (bottom left). The SEM sections clearly show the fenestrations in the tumor vasculature and their size relative to the plateloid particles (scale bar, 1.0 μm). d, Representative histological images of the tissue surrounding the tumor vasculature. The vessel wall is poorly organized with gaps between endothelial cells (top). The tumor cells are poorly packed, resulting in gaps between cells (bottom) (scale bar, 1.0 μm).
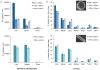
Figure 5. Sequestration of plateloid particles by immune cells
a, Percentage of particles co-localized with immune cells in different organs, measured using immunocytochemistry of fixed tissue cross-sections (Li: Liver; S: Spleen; Lu: Lungs; K: Kidneys; H: Heart and T: Tumor). b, Confocal fluorescence images of 1000×400 nm particles (red) co-localizing with CD204+ macrophages (blue) in fixed liver and spleen tissue. c, IHC images of 1000×400 nm (left) and 600×200 nm (right) particles in the liver and spleen. CD204+ macrophages are stained in brown. d, SEM images of 1000×400 nm particles in the liver vasculature being internalized by a Kupffer cell. A red blood cell (RBC) is visible at the bottom left of the larger image (scale bars, 5.0 and 1.0 μm). e, SEM images of 600×200 nm particles in the liver vasculature. The yellow arrows indicate particles that have been internalized; the white arrows indicate particles undergoing internalization (scale bars, 2.0 and 0.5 μm).
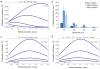
Figure 6. Adhesion of plateloid particles
a, The probability of adhesion (Pa) for plateloid particles is shown as a function of the diameter d and for different shear rates S (mr = 10+2 #/μm2; ml = 10+4 #/μm2, kao = 10−9 μm2); b, Number of plateloid particles adhering per unit area to the bottom of a parallel plate flow chamber, under different hydrodynamic conditions; c, Pa as a function of the particle diameter d and for different receptor densities mb (S = 100 sec−1 ; ml = 10+4 #/μm2, kao = 10−9 μm2); d, Pa as a function of the diameter d and for different ligand-receptor affinities kfo (S = 100 sec−1 ; mr = 10+2 #/μm2, ml = 10+4 #/μm2, kro=10−3 μm2).
Similar articles
- Investigation of silicon nanoparticles produced by centrifuge chemical vapor deposition for applications in therapy and diagnostics.
Lumen D, Wang S, Mäkilä E, Imlimthan S, Sarparanta M, Correia A, Westerveld Haug C, Hirvonen J, Santos HA, Airaksinen AJ, Filtvedt W, Salonen J. Lumen D, et al. Eur J Pharm Biopharm. 2021 Jan;158:254-265. doi: 10.1016/j.ejpb.2020.11.022. Epub 2020 Dec 3. Eur J Pharm Biopharm. 2021. PMID: 33279602 - Membrane Radiolabelling of Exosomes for Comparative Biodistribution Analysis in Immunocompetent and Immunodeficient Mice - A Novel and Universal Approach.
Faruqu FN, Wang JT, Xu L, McNickle L, Chong EM, Walters A, Gurney M, Clayton A, Smyth LA, Hider R, Sosabowski J, Al-Jamal KT. Faruqu FN, et al. Theranostics. 2019 Feb 28;9(6):1666-1682. doi: 10.7150/thno.27891. eCollection 2019. Theranostics. 2019. PMID: 31037130 Free PMC article. - Impact of surface grafting density of PEG macromolecules on dually fluorescent silica nanoparticles used for the in vivo imaging of subcutaneous tumors.
Adumeau L, Genevois C, Roudier L, Schatz C, Couillaud F, Mornet S. Adumeau L, et al. Biochim Biophys Acta Gen Subj. 2017 Jun;1861(6):1587-1596. doi: 10.1016/j.bbagen.2017.01.036. Epub 2017 Feb 4. Biochim Biophys Acta Gen Subj. 2017. PMID: 28179102 - The effect of nanoparticle properties, detection method, delivery route and animal model on poly(lactic-co-glycolic) acid nanoparticles biodistribution in mice and rats.
Simon LC, Sabliov CM. Simon LC, et al. Drug Metab Rev. 2014 May;46(2):128-41. doi: 10.3109/03602532.2013.864664. Epub 2013 Dec 5. Drug Metab Rev. 2014. PMID: 24303927 Review. - Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy.
Zhong Y, Meng F, Deng C, Zhong Z. Zhong Y, et al. Biomacromolecules. 2014 Jun 9;15(6):1955-69. doi: 10.1021/bm5003009. Epub 2014 May 12. Biomacromolecules. 2014. PMID: 24798476 Review.
Cited by
- Roadmap on nanomedicine.
Decuzzi P, Peer D, Mascolo DD, Palange AL, Manghnani PN, Moghimi SM, Farhangrazi ZS, Howard KA, Rosenblum D, Liang T, Chen Z, Wang Z, Zhu JJ, Gu Z, Korin N, Letourneur D, Chauvierre C, van der Meel R, Kiessling F, Lammers T. Decuzzi P, et al. Nanotechnology. 2021 Jan 1;32(1):012001. doi: 10.1088/1361-6528/abaadb. Nanotechnology. 2021. PMID: 33043901 Free PMC article. - Silicon micro- and nanofabrication for medicine.
Fine D, Grattoni A, Goodall R, Bansal SS, Chiappini C, Hosali S, van de Ven AL, Srinivasan S, Liu X, Godin B, Brousseau L 3rd, Yazdi IK, Fernandez-Moure J, Tasciotti E, Wu HJ, Hu Y, Klemm S, Ferrari M. Fine D, et al. Adv Healthc Mater. 2013 May;2(5):632-66. doi: 10.1002/adhm.201200214. Epub 2013 Apr 15. Adv Healthc Mater. 2013. PMID: 23584841 Free PMC article. Review. - Strategies for improving drug delivery: nanocarriers and microenvironmental priming.
Khalid A, Persano S, Shen H, Zhao Y, Blanco E, Ferrari M, Wolfram J. Khalid A, et al. Expert Opin Drug Deliv. 2017 Jul;14(7):865-877. doi: 10.1080/17425247.2017.1243527. Epub 2016 Oct 11. Expert Opin Drug Deliv. 2017. PMID: 27690153 Free PMC article. Review. - Transient mild hyperthermia induces E-selectin mediated localization of mesoporous silicon vectors in solid tumors.
Kirui DK, Mai J, Palange AL, Qin G, van de Ven AL, Liu X, Shen H, Ferrari M. Kirui DK, et al. PLoS One. 2014 Feb 18;9(2):e86489. doi: 10.1371/journal.pone.0086489. eCollection 2014. PLoS One. 2014. PMID: 24558362 Free PMC article. - Advancements in tantalum based nanoparticles for integrated imaging and photothermal therapy in cancer management.
Ifijen IH, Christopher AT, Lekan OK, Aworinde OR, Faderin E, Obembe O, Abdulsalam Akanji TF, Igboanugo JC, Udogu U, Ogidi GO, Iorkula TH, Osayawe OJ. Ifijen IH, et al. RSC Adv. 2024 Oct 23;14(46):33681-33740. doi: 10.1039/d4ra05732e. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39450067 Free PMC article. Review.
References
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;19:1818–1822. - PubMed
- Huang HC, Barua S, Sharma G, Dey SK, Rege K. Inorganic nanoparticles for cancer imaging and therapy. J Control Release. 2011 Jul 18; [e-published ahead of print] - PubMed
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nature Reviews Cancer. 2005;5:161–171. - PubMed
- Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnology. 2007;2:751–760. - PubMed
- Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjopoulos D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol Rev. 1999;51:691–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA143837-01/CA/NCI NIH HHS/United States
- U54 CA151668/CA/NCI NIH HHS/United States
- U54CA151668/CA/NCI NIH HHS/United States
- U54 CA143837/CA/NCI NIH HHS/United States
- U54 CA151668-01/CA/NCI NIH HHS/United States
- U54CA143837/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources