Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin - PubMed (original) (raw)
Existence of a novel clathrin-independent endocytic pathway in yeast that depends on Rho1 and formin
Derek C Prosser et al. J Cell Biol. 2011.
Abstract
Yeast is a powerful model organism for dissecting the temporal stages and choreography of the complex protein machinery during endocytosis. The only known mechanism for endocytosis in yeast is clathrin-mediated endocytosis, even though clathrin-independent endocytic pathways have been described in other eukaryotes. Here, we provide evidence for a clathrin-independent endocytic pathway in yeast. In cells lacking the clathrin-binding adaptor proteins Ent1, Ent2, Yap1801, and Yap1802, we identify a second endocytic pathway that depends on the GTPase Rho1, the downstream formin Bni1, and the Bni1 cofactors Bud6 and Spa2. This second pathway does not require components of the better-studied endocytic pathway, including clathrin and Arp2/3 complex activators. Thus, our results reveal the existence of a second pathway for endocytosis in yeast, which suggests similarities with the RhoA-dependent endocytic pathways of mammalian cells.
© 2011 Prosser et al.
Figures
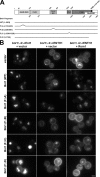
Figure 1.
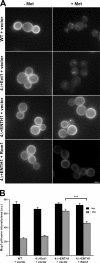
Suppression of temperature-dependent growth and endocytic defects by the Rho1 pathway. (A) Schematic of Rho1 pathway components. (B) Five-fold serial dilutions of WT, 4Δ+Ent1, and 4Δ+ENTH1 cells with empty vector, YAP1801, WSC1, MID2, ROM1, and RHO1 plasmids as indicated were grown at 30°C or 35.5°C. Growth at 35.5°C was scored as strong (++), moderate (+), or weak (−/+). (C) Cells from B, which express Ste3-GFP at endogenous levels, were examined by fluorescence microscopy. Insets show additional cells at the same magnification. Bar, 2.5 µm. (D) Intensity of Ste3-pHluorin was quantified in WT, 4Δ+Ent1, and 4Δ+ENTH1 cells expressing Ste3-pHluorin with the same plasmids used in B. Intensity values corrected for cell size are expressed in arbitrary units (a.u.; error bars indicate mean ± SEM; ***, P < 0.001 compared with all other conditions).
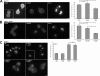
Figure 2.
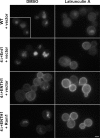
Effect of multicopy Rom1 on methionine-induced internalization of Mup1-pHluorin in adaptor mutant cells. (A) WT, 4Δ+Ent1, and 4Δ+ENTH1 cells with empty vector or Rom1 as indicated were grown in the absence of methionine to promote accumulation of Mup1 at the plasma membrane. Random fields of cells were imaged before treatment (−Met) or 30 min after addition of 20 µg/ml methionine (+Met) to the medium. For all images, identical maximum and minimum intensity values were applied to allow comparison of fluorescence intensity. Bar, 2.5 µm. (B) Quantification of Mup1-pHluorin intensity (a.u.; error bars indicate mean ± SEM; ***, P < 0.001) from the experiment in A. Black bars represent untreated (−Met) cells, whereas gray bars represent methionine-treated cells (+Met).
Figure 3.
The Rho1 pathway mediates actin-dependent internalization from the plasma membrane. WT, 4Δ+Ent1, and 4Δ+ENTH1 cells with empty vector or Rom1 were treated with 200 µM LatA or an equivalent volume of DMSO for 2 h before visualization of Ste3-GFP by fluorescence microscopy. The inset shows additional cells at the same magnification. Bar, 2.5 µm.
Figure 4.
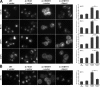
Effect of Rho1 effector deletion on endocytosis mediated by the Rho1 pathway. (A) Ste3-GFP was visualized by fluorescence microscopy in _sec3_Δ, _fks1_Δ, _skn7_Δ, and _bni1_Δ strains generated in WT, 4Δ+Ent1, and 4Δ+ENTH1 backgrounds with empty vector or Rom1 plasmids as indicated. (B) Ste3-GFP localization was assessed by fluorescence microscopy in _bnr1_Δ strains generated in WT, 4Δ+Ent1, and 4Δ+ENTH1 backgrounds with empty vector or Rom1 plasmids. Quantification of Ste3-pHluorin intensity (a.u.; error bars indicate mean ± SEM; ***, P < 0.001) is shown to the right of each group of images. Bars, 2.5 µm.
Figure 5.
Contribution of Bni1 protein–protein interaction regions to Rho1-mediated endocytosis. (A) Schematic of full-length and truncated Bni1 fragments used. Bni1 fragments were expressed from the BNI1 promoter. (B) Ste3-GFP was examined by fluorescence microscopy in _bni1_Δ 4Δ+Ent1 and _bni1_Δ 4Δ+ENTH1 cells with empty vector, full-length or truncated Bni1 fragments [_CEN TRP1_], and empty vector or high-copy Rom1 [2μ _URA3_] plasmids as indicated. Bar, 2.5 µm.
Figure 6.
Requirement of polarisome components for Rho1-mediated endocytosis. _spa2_Δ and _bud6_Δ strains were generated in WT, 4Δ+Ent1, and 4Δ+ENTH1 backgrounds with empty vector or Rom1 plasmids as indicated. Ste3-GFP localization was assessed by fluorescence microscopy. Quantification of Ste3-pHluorin intensity (a.u.; error bars indicate mean ± SEM) is shown to the right of each group of Ste3-GFP images. The inset shows additional cells at the same magnification. Bar, 2.5 µm.
Figure 7.
Requirement of actin cable stabilization, but not actin polymerization or stabilization at cortical patches, for Rho1-mediated endocytosis. (A and B) Ste3-GFP localization was assessed in _las17_Δ (A) and _sac6_Δ _scp1_Δ (B) cells with empty vector, Yap1801, or Rom1 plasmids. (C) _tpm1_Δ was generated in WT, 4Δ+Ent1, and 4Δ+ENTH1 cells, and Ste3-GFP localization was assessed in strains with empty vector or Rom1 as indicated. Quantification of Ste3-pHluorin intensity (a.u.; error bars indicate mean ± SEM; *, P < 0.05; ***, P < 0.001) is shown to the right of each group of Ste3-GFP images. Insets show additional cells at the same magnification. Bars, 2.5 µm.
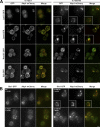
Figure 8.
Accumulation of actin module components in aberrant comet tail structures. (A) 4Δ+Ent1 and 4Δ+ENTH1 cells expressing GFP-tagged Las17, Sac6, Rvs167, or Sjl2 and Abp1-mCherry were assessed for colocalization of GFP (green) and mCherry (red) signals. Projection images from 0.25-µm-step z stacks are shown. (B) Projection images of WT, 4Δ+Ent1, and 4Δ+ENTH1 strains expressing Sla1-GFP and Abp1-mCherry with empty vector or Rom1 as indicated. Insets show additional cells at the same magnification. Bars, 2.5 µm.
Figure 9.
Rho1-mediated endocytosis does not restore cortical actin patch dynamics, and functions in the absence of clathrin. (A) Kymograph analysis of WT, 4Δ+Ent1, and 4Δ+ENTH1 cells expressing GFP-tagged Ede1, Pan1, or Las17 with empty vector or Rom1 as indicated. Images were collected every 4 s (480 s total) for Ede1, every 3 s (360 s total) for Pan1, and every 2 s (240 s total) for Las17. (B) Ste3-pHluorin localization was assessed in _chc1_Δ cells with empty vector, Yap1801, or Rom1 as indicated. Insets show additional cells at the same magnification. Bar, 2.5 µm. (C) Quantification of Ste3-pHluorin intensity (a.u.; error bars indicate mean ± SEM; ***, P < 0.001) in cells from B.
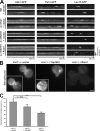
Figure 10.
Bni1-dependent suppression of endocytosis and growth defects in adaptor mutant cells grown with osmotic support. (A) WT, 4Δ+Ent1, and 4Δ+ENTH1 cells expressing Ste3-GFP were grown on synthetic medium in the absence (−sorbitol) or presence (+ 1 M sorbitol) of osmotic support as indicated and imaged by fluorescence microscopy. _bni1_Δ and _bnr1_Δ (B and C, respectively) in WT, 4Δ+Ent1, and 4Δ+ENTH1 adaptor backgrounds were assessed for Ste3-GFP localization in the absence or presence of osmotic support as in A. The inset shows additional cells at the same magnification. Bars, 2.5 µm. (D) Growth of strains used in A–C at permissive (30°C) and nonpermissive (37°C) temperatures in the absence (YPD) or presence (YPD + 1 M sorbitol) of osmotic support.
Similar articles
- Actin- and microtubule-based motors contribute to clathrin-independent endocytosis in yeast.
Woodard TK, Rioux DJ, Prosser DC. Woodard TK, et al. Mol Biol Cell. 2023 Nov 1;34(12):ar117. doi: 10.1091/mbc.E23-05-0164. Epub 2023 Aug 30. Mol Biol Cell. 2023. PMID: 37647159 Free PMC article. - Conserved roles for yeast Rho1 and mammalian RhoA GTPases in clathrin-independent endocytosis.
Prosser DC, Wendland B. Prosser DC, et al. Small GTPases. 2012 Oct-Dec;3(4):229-35. doi: 10.4161/sgtp.21631. Small GTPases. 2012. PMID: 23238351 Free PMC article. - In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast.
Newpher TM, Smith RP, Lemmon V, Lemmon SK. Newpher TM, et al. Dev Cell. 2005 Jul;9(1):87-98. doi: 10.1016/j.devcel.2005.04.014. Dev Cell. 2005. PMID: 15992543 - Functions of actin in endocytosis.
Robertson AS, Smythe E, Ayscough KR. Robertson AS, et al. Cell Mol Life Sci. 2009 Jul;66(13):2049-65. doi: 10.1007/s00018-009-0001-y. Epub 2009 Mar 17. Cell Mol Life Sci. 2009. PMID: 19290477 Free PMC article. Review. - Molecular requirements for the internalisation step of endocytosis: insights from yeast.
Munn AL. Munn AL. Biochim Biophys Acta. 2001 Mar 26;1535(3):236-57. doi: 10.1016/s0925-4439(01)00028-x. Biochim Biophys Acta. 2001. PMID: 11278164 Review.
Cited by
- Rab5-mediated endosome formation is regulated at the _trans_-Golgi network.
Nagano M, Toshima JY, Siekhaus DE, Toshima J. Nagano M, et al. Commun Biol. 2019 Nov 15;2:419. doi: 10.1038/s42003-019-0670-5. eCollection 2019. Commun Biol. 2019. PMID: 31754649 Free PMC article. - Emerging Mechanisms of Endocytosis in Toxoplasma gondii.
McGovern OL, Rivera-Cuevas Y, Carruthers VB. McGovern OL, et al. Life (Basel). 2021 Jan 25;11(2):84. doi: 10.3390/life11020084. Life (Basel). 2021. PMID: 33503859 Free PMC article. Review. - Pan1 regulates transitions between stages of clathrin-mediated endocytosis.
Bradford MK, Whitworth K, Wendland B. Bradford MK, et al. Mol Biol Cell. 2015 Apr 1;26(7):1371-85. doi: 10.1091/mbc.E14-11-1510. Epub 2015 Jan 28. Mol Biol Cell. 2015. PMID: 25631817 Free PMC article. - Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis.
Zhu YH, Hyun J, Pan YZ, Hopper JE, Rizo J, Wu JQ. Zhu YH, et al. Mol Biol Cell. 2018 Sep 15;29(19):2259-2279. doi: 10.1091/mbc.E18-04-0225. Epub 2018 Jul 25. Mol Biol Cell. 2018. PMID: 30044717 Free PMC article. - CAP-Gly proteins contribute to microtubule-dependent trafficking via interactions with the C-terminal aromatic residue of α-tubulin.
Andrieux A, Aubry L, Boscheron C. Andrieux A, et al. Small GTPases. 2019 Mar;10(2):138-145. doi: 10.1080/21541248.2016.1277002. Epub 2017 Jan 27. Small GTPases. 2019. PMID: 28103137 Free PMC article. Review.
References
- Aguilar R.C., Longhi S.A., Shaw J.D., Yeh L.Y., Kim S., Schön A., Freire E., Hsu A., McCormick W.K., Watson H.A., Wendland B. 2006. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA. 103:4116–4121 10.1073/pnas.0510513103 - DOI - PMC - PubMed
- Ayscough K.R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D.G. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416 10.1083/jcb.137.2.399 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases