Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress - PubMed (original) (raw)
Human umbilical cord blood-derived mesenchymal stem cells undergo cellular senescence in response to oxidative stress
Eun Ko et al. Stem Cells Dev. 2012.
Abstract
Since human mesenchymal stem cells (MSCs) are therapeutically attractive for tissue regeneration and repair, we examined the physiological responses of human umbilical cord blood-derived MSCs (hUCB-MSCs) to genotoxic stress. We found that that sublethal doses of reactive oxygen species (ROS) and ionizing radiation cause DNA damage and reduce DNA synthesis and cell proliferation in hUCB-MSCs, resulting in cellular senescence. In contrast, these physiological changes were limited in human fibroblast and cancer cells. Our data show that reduced activities of antioxidant enzymes, which may occur due to low gene expression levels, cause hUCB-MSCs to undergo cellular senescence in response to oxidative stress and ionizing radiation. Resistance of hUCB-MSCs to oxidative stresses was restored by increasing the intracellular antioxidant activity in hUCB-MSCs via exogenous addition of antioxidants. Therefore, the proliferation and fate of hUCB-MSCs can be controlled by exposure to oxidative stresses.
Figures
FIG. 1.
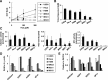
Proliferation of hUCB-MSCs is susceptible to genotoxic stresses. (A, B) hUCB-MSCs (MSC1, 2, 3, and 4), human fibroblast cells (MRC5 and HS68), and cancer cells (U2OS and HeLa) were treated with the indicated concentrations of hydrogen peroxide for 2 h as previously described [22]. The cells were washed twice with PBS and further incubated in fresh medium for 24 h, followed by immunostaining with the anti-Ki-67 (see also Supplementary Fig. S1) or anti-BrdU antibody for detection of cell proliferation or DNA synthesis, respectively. (C, D) Cells irradiated with γ-rays were grown for 24 h and immunostained as described previously. For the BrdU incorporation assay, cells were incubated for 30 min in the presence of 10 μM of BrdU prior to immunostaining as previously described [22]. DNA was visualized using DAPI staining. The proportion of Ki-67 or BrdU-positive cells was determined in each untreated or treated cell population; then the fold difference in proportion between treated and untreated cells is described on graphs as “Proliferation” or “DNA synthesis,” respectively. Graphs represent the averages of at least 3 independent experiments, detecting more than 100 cells each. The percentages (%) of untreated cells exhibiting Ki-67–positive staining were as follows: 90.5, U2OS; 87.5, HeLa; 73.9, HS68; 83.5, MRC5; 82.0, MSC1; 81.0, MSC2; 69.4, MSC3; and 58.8, MSC4. The percentages (%) of untreated cells exhibiting BrdU-positive staining were as follows: 33.3, U2OS; 37.3, HeLa; 36, HS68; 33.7, MRC5; 30.7, MSC1; 30.0, MSC2; 25.3, MSC3; and 28.5, MSC4. The P values were <0.0001 (A), <0.0001 (B), 0.0023 (C), and 0.0004 (D). MSCs, mesenchymal stem cells; hUCB-MSCs, human umbilical cord blood–derived MSCs; PBS, phosphate-buffered saline.
FIG. 2.
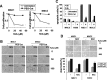
Oxidative stress causes more DNA breaks in hUCB-MSCs. (A, B) Cells incubated with the indicated concentration of hydrogen peroxide for 30 min (A) or 2 h (B) were analyzed in a comet assay to measure DNA damage. (C) The olive tail moment values of the 2-h samples were measured using the Komet 7.0 software and indicated as previously described [52,53]. The P value was 0.0124.
FIG. 3.
Recovery following DNA damage in hUCB-MSCs. (A, C) Cells treated with the indicated concentration of hydrogen peroxide for 30 min were washed with PBS and then incubated in fresh media for the indicated number of hours. Comet assays were then performed. Values of olive tail moment after treatment with 500 μM (B) or 50 μM (D) of hydrogen peroxide are presented. The P values were <0.0001 **(B)** and >0.05 (D).
FIG. 4.
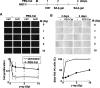
Oxidative stress induces cellular senescence in hUCB-MSCs. (A) Cells incubated with 0 or 100 μM of hydrogen peroxide for 2 h were washed with PBS, and then incubated in fresh medium for 4 days. Cells were then stained with SA-β-Gal. (B) The percentage of cells stained blue (SA-β-Gal positive) is indicated. The data represent the average of 3 independent experiments, using over 150 cells each. (C) Cells incubated with 200 or 500 μM of hydrogen peroxide for 2 or 4 h were stained with trypan blue, and dead cells were scored using a hemocytometer. (D) Cells treated with 10 μM of phorbol-12-myristate-13-acetate for 4 h (see also Supplementary Fig. S5) were stained with trypan blue solution. The P values were <0.001 (B) and 0.0047 (D). SA-β-Gal, senescence-associated β-galactosidase.
FIG. 5.
hUCB-MSCs show low antioxidant activity. (A) Cells incubated with 20 μM of 2′,7′-dichlorodihydrofluorescein diacetate for 30 min were washed, and then incubated with fresh media containing the indicated concentration of hydrogen peroxide for 30 min. Intracellular ROS levels were detected using the FACS Calibur instrument (BD Bioscience). Mean fluorescence intensity of DCF was used as measure of ROS, and values were normalized with the basal intensity of each cell line. (B) Total antioxidant activity in each cell lysate was measured using Trolox E as described in the Materials and Methods section. Values per 1 μg of cell lysate are shown. (C) Activities of catalase, superoxide dismutase (SOD), and glutathione peroxidase (GPx) were measured as described in the Materials and Methods section. Enzyme activity per 1 μg of cell lysate is indicated. (D) 40 μg of total protein from each cell line was immunoblotted with the appropriate antibodies (see also Supplementary Fig. S6A). Relative band intensities normalized to the β-actin band were calculated using Multi Gauge 3.0 software (Fujifilm). SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; GPx1, glutathione peroxidase 1. (E) 1 μg of mRNA from each cell line was reverse transcribed using specific primers (Materials and Methods). Relative band intensities from gel electrophoresis following RT-PCR (Supplementary Fig. S6B) were measured using Multi Gauge 3.0 software. Quantitative data for each RT-PCR product were normalized against the GAPDH level. The P values were 0.036 (A); 0.017 (B); 0.0081, catalase activity in (C); 0.0076, SOD activity in (C); and <0.0001, GPx activity in (C). DCF, 2′,7′-dichlorodihydrofluorescein; ROS, reactive oxygen species.
FIG. 6.
Exogenous addition of antioxidant prevents hUCB-MSCs from undergoing cellular senescence and cell death due to oxidative stress. (A) Cells incubated with 200 U/mL of PEG-catalase (PEG-Cat) for 24 h were further incubated for 2 h in fresh medium containing the indicated concentrations of hydrogen peroxide. The cells were then transferred to fresh medium, grown for 24 h, and immunostained using anti-Ki-67 antibodies (see also Supplementary Fig. S7A, B). (B) Cells pretreated with 200 U/mL of PEG-catalase were incubated in fresh medium for 2 h with hydrogen peroxide. Four days after hydrogen peroxide treatment, the cells were stained using SA-β-Gal. The percentage of SA-β-Gal–positive cells is indicated in each micrograph. (C) PEG-catalase (200 U/mL)–pretreated cells were incubated with 500 μM of hydrogen peroxide for 2 or 4 h. Cells were observed under a phase-contrast microscope (Supplementary Fig. S7C), and harvested cells were stained with trypan blue solution and counted. (D) hUCB-MSC lines, MSC1 and MSC2, were incubated with 1 mM of _N_-acetyl cysteine (NAC) for 6 h, and then incubated for 2 h in fresh medium containing the indicated concentrations of hydrogen peroxide. Cells were viewed under a phase-contrast microscope, and dead cells were counted using trypan blue staining. The graph shows the results from 3 independent experiments. The P values were <0.0001 (C) and 0.011 (D). PEG-catalase, polyethylene glycol–conjugated catalase.
FIG. 7.
Increased antioxidant activity inhibits hUCB-MSCs from undergoing cellular senescence following ionizing radiation. (A) MSC1 hUCB-MSCs, pretreated with 200 U/mL of PEG-catalase for 24 h, were irradiated with γ-rays. One day after irradiation, cells were immunostained using the anti-Ki-67 antibody (upper panel). DAPI staining was used to visualize nuclei. The relative proportion of Ki-67–stained cells is shown in the plot (lower panel). The results of 3 independent experiments were averaged. P value was 0.037. (B) Two or 4 days after irradiation, MSC1 cells were stained using SA-β-Gal and viewed under a phase-contrast microscope (upper panel). The graph represents the percentage of SA-β-Gal–stained cells at 4 days after irradiation (lower panel). IR, γ-irradiation.
Similar articles
- Downregulation of Melanoma Cell Adhesion Molecule (MCAM/CD146) Accelerates Cellular Senescence in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells.
Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ, Oh W, Yang YS, Jeon HB. Jin HJ, et al. Stem Cells Transl Med. 2016 Apr;5(4):427-39. doi: 10.5966/sctm.2015-0109. Epub 2016 Mar 3. Stem Cells Transl Med. 2016. PMID: 26941359 Free PMC article. - Therapeutic effect of human umbilical cord blood mesenchymal stem cells combined with G-CSF on rats with acute liver failure.
Chen H, Tang S, Liao J, Liu M, Lin Y. Chen H, et al. Biochem Biophys Res Commun. 2019 Oct 1;517(4):670-676. doi: 10.1016/j.bbrc.2019.07.101. Epub 2019 Aug 7. Biochem Biophys Res Commun. 2019. PMID: 31400854 - SIRT1-dependent anti-senescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells.
Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, Yang H, Pei M, He F. Zhou L, et al. J Tissue Eng Regen Med. 2018 Feb;12(2):e1008-e1021. doi: 10.1002/term.2422. Epub 2017 Jun 20. J Tissue Eng Regen Med. 2018. PMID: 28107614 Free PMC article. - The emerging antioxidant paradigm of mesenchymal stem cell therapy.
Stavely R, Nurgali K. Stavely R, et al. Stem Cells Transl Med. 2020 Sep;9(9):985-1006. doi: 10.1002/sctm.19-0446. Epub 2020 Jun 4. Stem Cells Transl Med. 2020. PMID: 32497410 Free PMC article. Review. - Microbial Stresses on Human Umbilical Cord Stem Cells.
Kart D, Çelebi-Saltik B. Kart D, et al. Curr Stem Cell Res Ther. 2021;16(7):801-808. doi: 10.2174/1574888X15666200309144334. Curr Stem Cell Res Ther. 2021. PMID: 32148203 Review.
Cited by
- Can mobilization of bone marrow stem cells be an alternative regenerative therapy to stem cell injection in a rat model of chronic kidney disease?
Morsy S, Mansour MF, Abdo M, El-Wazir Y. Morsy S, et al. Physiol Rep. 2022 Sep;10(17):e15448. doi: 10.14814/phy2.15448. Physiol Rep. 2022. PMID: 36065849 Free PMC article. - Effects of Oxidative Stress on Mesenchymal Stem Cell Biology.
Denu RA, Hematti P. Denu RA, et al. Oxid Med Cell Longev. 2016;2016:2989076. doi: 10.1155/2016/2989076. Epub 2016 Jun 16. Oxid Med Cell Longev. 2016. PMID: 27413419 Free PMC article. Review. - Computational comparative analysis identifies potential stemness-related markers for mesenchymal stromal/stem cells.
Ghabriel M, El Hosseiny A, Moustafa A, Amleh A. Ghabriel M, et al. Front Cell Dev Biol. 2023 Mar 1;11:1065050. doi: 10.3389/fcell.2023.1065050. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36936690 Free PMC article. - A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration.
Wang Y, Chen S, Yan Z, Pei M. Wang Y, et al. Cell Biosci. 2019 Jan 5;9:7. doi: 10.1186/s13578-018-0264-9. eCollection 2019. Cell Biosci. 2019. PMID: 30627420 Free PMC article. Review. - Dysfunction of Mesenchymal Stem Cells Isolated from Metabolic Syndrome and Type 2 Diabetic Patients as Result of Oxidative Stress and Autophagy may Limit Their Potential Therapeutic Use.
Kornicka K, Houston J, Marycz K. Kornicka K, et al. Stem Cell Rev Rep. 2018 Jun;14(3):337-345. doi: 10.1007/s12015-018-9809-x. Stem Cell Rev Rep. 2018. PMID: 29611042 Free PMC article. Review.
References
- Hussain SP. Hofseth LJ. Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. - PubMed
- Helleday T. Petermann E. Lundin C. Hodgson B. Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. - PubMed
- Branzei D. Foiani M. Regulation of DNA repair throughout the cell cycle. Nat Rev Mol Cell Biol. 2008;9:297–308. - PubMed
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. - PubMed
- d'Adda di Fagagna F. Living on a break: cellular senescence as a DNA-damage response. Nat Rev Cancer. 2008;8:512–522. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources