Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention - PubMed (original) (raw)
Multicenter Study
doi: 10.1016/j.annemergmed.2011.08.021. Epub 2011 Nov 8.
Lawrence M Lewis, Jay L Falk, Zhiqun Zhang, Salvatore Silvestri, Philip Giordano, Gretchen M Brophy, Jason A Demery, Neha K Dixit, Ian Ferguson, Ming Cheng Liu, Jixiang Mo, Linnet Akinyi, Kara Schmid, Stefania Mondello, Claudia S Robertson, Frank C Tortella, Ronald L Hayes, Kevin K W Wang
Affiliations
- PMID: 22071014
- PMCID: PMC3830977
- DOI: 10.1016/j.annemergmed.2011.08.021
Multicenter Study
Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention
Linda Papa et al. Ann Emerg Med. 2012 Jun.
Abstract
Study objective: This study examines whether serum levels of glial fibrillary acidic protein breakdown products (GFAP-BDP) are elevated in patients with mild and moderate traumatic brain injury compared with controls and whether they are associated with traumatic intracranial lesions on computed tomography (CT) scan (positive CT result) and with having a neurosurgical intervention.
Methods: This prospective cohort study enrolled adult patients presenting to 3 Level I trauma centers after blunt head trauma with loss of consciousness, amnesia, or disorientation and a Glasgow Coma Scale (GCS) score of 9 to 15. Control groups included normal uninjured controls and trauma controls presenting to the emergency department with orthopedic injuries or a motor vehicle crash without traumatic brain injury. Blood samples were obtained in all patients within 4 hours of injury and measured by enzyme-linked immunosorbent assay for GFAP-BDP (nanograms/milliliter).
Results: Of the 307 patients enrolled, 108 were patients with traumatic brain injury (97 with GCS score 13 to 15 and 11 with GCS score 9 to 12) and 199 were controls (176 normal controls and 16 motor vehicle crash controls and 7 orthopedic controls). Receiver operating characteristic curves demonstrated that early GFAP-BDP levels were able to distinguish patients with traumatic brain injury from uninjured controls with an area under the curve of 0.90 (95% confidence interval [CI] 0.86 to 0.94) and differentiated traumatic brain injury with a GCS score of 15 with an area under the curve of 0.88 (95% CI 0.82 to 0.93). Thirty-two patients with traumatic brain injury (30%) had lesions on CT. The area under these curves for discriminating patients with CT lesions versus those without CT lesions was 0.79 (95% CI 0.69 to 0.89). Moreover, the receiver operating characteristic curve for distinguishing neurosurgical intervention from no neurosurgical intervention yielded an area under the curve of 0.87 (95% CI 0.77 to 0.96).
Conclusion: GFAP-BDP is detectable in serum within an hour of injury and is associated with measures of injury severity, including the GCS score, CT lesions, and neurosurgical intervention. Further study is required to validate these findings before clinical application.
Copyright © 2012. Published by Mosby, Inc.
Figures
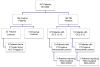
Figure 1. Flow diagram of enrolled patients
Flow diagram showing the number of all TBI and control patients enrolled.
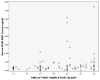
Figure 2. Temporal profile of GFAP-BDP in TBI patients within 4 hours of injury
The dots represent levels of GFAP-BDP (ng/ml) at different times post-injury. GFAP-BDP demonstrated a rapid appearance in serum post-injury with levels detectible within an hour of injury.
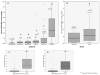
Figures 3a, 3b, 3c, & 3d
Figure 3a. A comparison of serum levels of GFAP-BDP drawn within 4 hours of injury in TBI patients with different GCS scores versus normal and trauma controls. GCS scores were divided as GCS 15, GCS 13–14, and GCS 9–12. There are 3 control groups: i- non-injured, ii- non-head injured orthopedic controls, and iii- non-head injured controls from motor vehicle collisions. There are statistically significant differences between the uninjured controls and each of the groups (indicated by the asterisk “*”) and serum levels of GFAP-BDP increased incrementally with worsening GCS scores. Boxplots represent medians in ng/ml and interquartile ranges. Figure 3b. A comparison of serum levels of GFAP-BDP drawn within 4 hours of injury in TBI patients with GCS 15 versus trauma controls. When TBI patients with an ED GCS score of 15 are isolated from the TBI group early GFAP-BDP levels demonstrate significant differences between patients with a GCS 15 versus trauma controls. Boxplots represent medians in ng/ml and interquartile ranges. Figure 3c. A comparison of serum levels of GFAP-BDP in TBI patients with GCS dichotomized into GCS 13–15 versus GCS 9–12. When TBI patients are dichotomized into the traditional GCS 13–15 versus GCS 9–12, early GFAP-BDP levels are significantly different between the 2 groups. Boxplots represent medians in ng/ml and interquartile ranges. Figure 3d. A comparison of serum levels of GFAP-BDP in TBI patients with GCS dichotomized into GCS 14–15 versus GCS 9–13. When TBI patients are dichotomized into the more recently suggested dichotomy of GCS 14–15 versus GCS 9–13, early GFAP-BDP levels are significantly different between the 2 groups. Boxplots represent medians in ng/ml and interquartile ranges. * Statistically significant difference
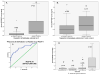
Figures 4a & 4b
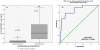
Figure 4a. ROC Curve for distinguishing TBI versus uninjured controls. The area under the ROC curve (AUC) demonstrates that early GFAP-BDP levels are able discriminate between patients without injuries from patients with TBI with an AUC 0.90 (95%CI 0.86–0.94). Figure 4b. ROC Curve for distinguishing TBI patients with a GCS 15 versus uninjured controls. TBI Patients with a GCS 15 are isolated from the group and compared to uninjured controls. Early GFAP-BDP levels are able discriminate between patients without injuries from TBI patients with a GCS 15 with an AUC 0.88 (95%CI 0.82–0.93).
Figures 5a, 5b & 5c
Figure 5a. Boxlplot comparing serum GFAP-BDP levels drawn within 4 hours of injury in patients with and without traumatic intracranial lesions on CT. Levels of serum GFAP-BDP are significantly higher in patients with traumatic intracranial lesions on CT (CT positive) than those without CT lesions (CT negative). Bars represent medians in ng/ml and interquartile ranges. Figure 5b. Boxplot comparing serum GFAP-BDP levels drawn within 4 hours of injury in patients with and without traumatic intracranial lesions on CT in TBI patients with GCS 15. In a subset of TBI patients with a GCS 15 levels of serum GFAP-BDP are significantly higher in those with traumatic intracranial lesions on CT (CT positive) than those without CT lesions (CT negative). Bars represent medians in ng/ml and interquartile ranges. Figure 5c. ROC Curve for distinguishing CT positive versus CT negative patients. The area under the ROC curve (AUC) demonstrates that early GFAP-BDP levels are able discriminate between patients with and without traumatic intracranial lesions on CT (AUC 0.79; 95%CI 0.69–0.89). Figure 5d. Boxplot comparing serum GFAP-BDP levels drawn within 4 hours of injury in patients with and without traumatic intracranial lesions on CT in both TBI and trauma controls. GFAP-BDP levels are significantly higher in patients with traumatic intracranial lesions on CT (CT positive) than those without CT lesions (CT negative) regardless of whether they are trauma controls or TBI. Bars represent medians in ng/ml and interquartile ranges. * Statistically significant difference
Figures 6a, 6b
Figure 6a. Boxplot comparing serum GFAP-BDP levels drawn within 4 hours of injury in patients with neurosurgical intervention versus those without intervention. Levels of serum GFAP-BDP are significantly higher in patients with who had neurosurgical intervention versus those who did not. Bars represent medians in ng/ml and interquartile ranges. Figure 6b. ROC Curve for distinguishing those who had a neurosurgical intervention versus those who did not. The area under the ROC curve (AUC) demonstrates that early GFAP-BDP levels are able discriminate between patients with who required neurosurgical intervention versus those who did not (AUC 0.87; 95%CI 0.77–0.96). * Statistically significant difference
Similar articles
- Performance of Glial Fibrillary Acidic Protein in Detecting Traumatic Intracranial Lesions on Computed Tomography in Children and Youth With Mild Head Trauma.
Papa L, Zonfrillo MR, Ramirez J, Silvestri S, Giordano P, Braga CF, Tan CN, Ameli NJ, Lopez M, Mittal MK. Papa L, et al. Acad Emerg Med. 2015 Nov;22(11):1274-82. doi: 10.1111/acem.12795. Epub 2015 Oct 15. Acad Emerg Med. 2015. PMID: 26469937 Free PMC article. - Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention.
Papa L, Lewis LM, Silvestri S, Falk JL, Giordano P, Brophy GM, Demery JA, Liu MC, Mo J, Akinyi L, Mondello S, Schmid K, Robertson CS, Tortella FC, Hayes RL, Wang KK. Papa L, et al. J Trauma Acute Care Surg. 2012 May;72(5):1335-44. doi: 10.1097/TA.0b013e3182491e3d. J Trauma Acute Care Surg. 2012. PMID: 22673263 Free PMC article. Clinical Trial. - Measurement of the glial fibrillary acidic protein and its breakdown products GFAP-BDP biomarker for the detection of traumatic brain injury compared to computed tomography and magnetic resonance imaging.
McMahon PJ, Panczykowski DM, Yue JK, Puccio AM, Inoue T, Sorani MD, Lingsma HF, Maas AI, Valadka AB, Yuh EL, Mukherjee P, Manley GT, Okonkwo DO; TRACK-TBI Investigators. McMahon PJ, et al. J Neurotrauma. 2015 Apr 15;32(8):527-33. doi: 10.1089/neu.2014.3635. Epub 2015 Jan 26. J Neurotrauma. 2015. PMID: 25264814 Free PMC article. Clinical Trial. - Will Neuroimaging Reveal a Severe Intracranial Injury in This Adult With Minor Head Trauma?: The Rational Clinical Examination Systematic Review.
Easter JS, Haukoos JS, Meehan WP, Novack V, Edlow JA. Easter JS, et al. JAMA. 2015 Dec 22-29;314(24):2672-81. doi: 10.1001/jama.2015.16316. JAMA. 2015. PMID: 26717031 Review. - Serum Glial Fibrillary Acidic Protein in Detecting Intracranial Injuries Following Minor Head Trauma; a Systematic Review and Meta-Analysis.
Ahmadi S, Roshdi Dizaji S, Babahajian A, Alizadeh M, Sarveazad A, Yousefifard M. Ahmadi S, et al. Arch Acad Emerg Med. 2023 Jan 1;11(1):e9. doi: 10.22037/aaem.v11i1.1682. eCollection 2023. Arch Acad Emerg Med. 2023. PMID: 36620734 Free PMC article. Review.
Cited by
- Systems biology approaches for discovering biomarkers for traumatic brain injury.
Feala JD, Abdulhameed MD, Yu C, Dutta B, Yu X, Schmid K, Dave J, Tortella F, Reifman J. Feala JD, et al. J Neurotrauma. 2013 Jul 1;30(13):1101-16. doi: 10.1089/neu.2012.2631. J Neurotrauma. 2013. PMID: 23510232 Free PMC article. Review. - Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker.
Yang Z, Wang KK. Yang Z, et al. Trends Neurosci. 2015 Jun;38(6):364-74. doi: 10.1016/j.tins.2015.04.003. Epub 2015 May 11. Trends Neurosci. 2015. PMID: 25975510 Free PMC article. Review. - Current status of fluid biomarkers in mild traumatic brain injury.
Kulbe JR, Geddes JW. Kulbe JR, et al. Exp Neurol. 2016 Jan;275 Pt 3(0 3):334-352. doi: 10.1016/j.expneurol.2015.05.004. Epub 2015 May 14. Exp Neurol. 2016. PMID: 25981889 Free PMC article. Review. - Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury.
Yue JK, Vassar MJ, Lingsma HF, Cooper SR, Okonkwo DO, Valadka AB, Gordon WA, Maas AI, Mukherjee P, Yuh EL, Puccio AM, Schnyer DM, Manley GT; TRACK-TBI Investigators. Yue JK, et al. J Neurotrauma. 2013 Nov 15;30(22):1831-44. doi: 10.1089/neu.2013.2970. Epub 2013 Sep 24. J Neurotrauma. 2013. PMID: 23815563 Free PMC article. Clinical Trial. - Sympathoadrenal Activation is Associated with Acute Traumatic Coagulopathy and Endotheliopathy in Isolated Brain Injury.
Di Battista AP, Rizoli SB, Lejnieks B, Min A, Shiu MY, Peng HT, Baker AJ, Hutchison MG, Churchill N, Inaba K, Nascimento BB, de Oliveira Manoel AL, Beckett A, Rhind SG. Di Battista AP, et al. Shock. 2016 Sep;46(3 Suppl 1):96-103. doi: 10.1097/SHK.0000000000000642. Shock. 2016. PMID: 27206278 Free PMC article.
References
- Consensus conference. Rehabilitation of persons with traumatic brain injury. NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury. Jama. 1999;282(10):974–983. - PubMed
- Faul M, Xu L, Wald MM, Coronado VG. Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2010. [Accessed November 11, 2010]. Traumatic Brain Injury in the United States. http://www.cdc.gov/traumaticbraininjury/pdf/blue_book.pdf.
- Carey ME. Analysis of wounds incurred by U.S. Army Seventh Corps personnel treated in Corps hospitals during Operation Desert Storm, February 20 to March 10, 1991. J Trauma. 1996 Mar;40(3 Suppl):S165–169. - PubMed
- Sapsford W. Penetrating brain injury in military conflict: does it merit more research? J R Army Med Corps. 2003 Mar;149(1):5–14. - PubMed
- Okie S. Traumatic brain injury in the war zone. N Engl J Med. 2005 May 19;352(20):2043–2047. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous