Predicting the effect of climate change on African trypanosomiasis: integrating epidemiology with parasite and vector biology - PubMed (original) (raw)
Predicting the effect of climate change on African trypanosomiasis: integrating epidemiology with parasite and vector biology
Sean Moore et al. J R Soc Interface. 2012.
Abstract
Climate warming over the next century is expected to have a large impact on the interactions between pathogens and their animal and human hosts. Vector-borne diseases are particularly sensitive to warming because temperature changes can alter vector development rates, shift their geographical distribution and alter transmission dynamics. For this reason, African trypanosomiasis (sleeping sickness), a vector-borne disease of humans and animals, was recently identified as one of the 12 infectious diseases likely to spread owing to climate change. We combine a variety of direct effects of temperature on vector ecology, vector biology and vector-parasite interactions via a disease transmission model and extrapolate the potential compounding effects of projected warming on the epidemiology of African trypanosomiasis. The model predicts that epidemics can occur when mean temperatures are between 20.7°C and 26.1°C. Our model does not predict a large-range expansion, but rather a large shift of up to 60 per cent in the geographical extent of the range. The model also predicts that 46-77 million additional people may be at risk of exposure by 2090. Future research could expand our analysis to include other environmental factors that influence tsetse populations and disease transmission such as humidity, as well as changes to human, livestock and wildlife distributions. The modelling approach presented here provides a framework for using the climate-sensitive aspects of vector and pathogen biology to predict changes in disease prevalence and risk owing to climate change.
Figures
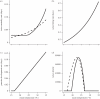
Figure 1.
Relationship between temperature and four different parameters that influence _R_0. The (a) daily mortality rate of G. m. morsitans (dashed line) and G. pallidipes (_d_V, solid line), (b) tsetse daily feeding rate a. (c) Daily rate of parasite development in tsetse (μ) and (d) G. m. morsitans and G. pallidipes abundance (_N_V) as a function of mean annual temperature.
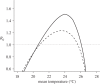
Figure 2.
Relationship between temperature and _R_0 when T. b. rhodesiense is vectored by G. m. morsitans (dashed line) or G. pallidipes (solid line). _R_0 = 1 represents a threshold for the successful invasion or persistence of the parasite into a susceptible host community.
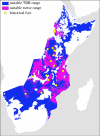
Figure 3.
Suitable geographical range for T. b. rhodesiense transmission based on range where _R_0 > 1 for G. pallidipes. The darker grey region (purple on the online version) represents the portion of the range also predicted to be the ideal habitat for Morsitans group tsetse flies. Lighter grey (blue on the online version) represents the portion of the suitable range currently believed to be unoccupied by Morsitans group tsetse flies. Circles represent locations of previous African trypanosomiasis outbreaks in East Africa [62]. (Online version in colour.)
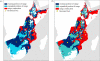
Figure 4.
Suitable geographical range for T. b. rhodesiense transmission in (a) 2055 and (b) 2090 under the A2 emissions scenario using the CCSM3 global circulation model. The predicted range is in black (light blue on the online version), with the white stippled (dark blue on the online version) region representing the existing portion of the predicted range and the unstippled area representing the newly expanded part of the range. Grey (red on the online version) areas on the map represent currently suitable areas predicted to be too hot under future conditions. Circles represent locations of previous outbreaks in East Africa [62]. (Online version in colour.)
Similar articles
- Modelling the impact of climate change on the distribution and abundance of tsetse in Northern Zimbabwe.
Longbottom J, Caminade C, Gibson HS, Weiss DJ, Torr S, Lord JS. Longbottom J, et al. Parasit Vectors. 2020 Oct 19;13(1):526. doi: 10.1186/s13071-020-04398-3. Parasit Vectors. 2020. PMID: 33076987 Free PMC article. - Potential impacts of climate change on geographical distribution of three primary vectors of African Trypanosomiasis in Tanzania's Maasai Steppe: G. m. morsitans, G. pallidipes and G. swynnertoni.
Nnko HJ, Gwakisa PS, Ngonyoka A, Sindato C, Estes AB. Nnko HJ, et al. PLoS Negl Trop Dis. 2021 Feb 11;15(2):e0009081. doi: 10.1371/journal.pntd.0009081. eCollection 2021 Feb. PLoS Negl Trop Dis. 2021. PMID: 33571190 Free PMC article. - Adult blood-feeding tsetse flies, trypanosomes, microbiota and the fluctuating environment in sub-Saharan Africa.
Geiger A, Ponton F, Simo G. Geiger A, et al. ISME J. 2015 Jul;9(7):1496-507. doi: 10.1038/ismej.2014.236. Epub 2014 Dec 12. ISME J. 2015. PMID: 25500509 Free PMC article. Review. - Satellites, space, time and the African trypanosomiases.
Rogers DJ. Rogers DJ. Adv Parasitol. 2000;47:129-71. doi: 10.1016/s0065-308x(00)47008-9. Adv Parasitol. 2000. PMID: 10997206 Review. - Know your foe: lessons from the analysis of tsetse fly behaviour.
Torr SJ, Vale GA. Torr SJ, et al. Trends Parasitol. 2015 Mar;31(3):95-9. doi: 10.1016/j.pt.2014.12.010. Epub 2015 Jan 15. Trends Parasitol. 2015. PMID: 25599585 Review.
Cited by
- [Climate-sensitive diseases in Brazil and the world: systematic reviewEnfermedades sensibles al clima en Brasil y el mundo: revisión sistemática].
de Sousa TCM, Amancio F, Hacon SS, Barcellos C. de Sousa TCM, et al. Rev Panam Salud Publica. 2018 Jul 20;42:e85. doi: 10.26633/RPSP.2018.85. eCollection 2018. Rev Panam Salud Publica. 2018. PMID: 31093113 Free PMC article. Review. Portuguese. - Impact of climate change on occupational health and productivity: a systematic literature review focusing on workplace heat.
Levi M, Kjellstrom T, Baldasseroni A. Levi M, et al. Med Lav. 2018 Apr 24;109(3):163-79. doi: 10.23749/mdl.v109i3.6851. Med Lav. 2018. PMID: 29943748 Free PMC article. Review. - Coinfection With Trypanosoma brucei Confers Protection Against Cutaneous Leishmaniasis.
Pereira L, Oliveira F, Townsend S, Metangmo S, Meneses C, Moore IN, Brodskyn CI, Valenzuela JG, Magez S, Kamhawi S. Pereira L, et al. Front Immunol. 2018 Dec 11;9:2855. doi: 10.3389/fimmu.2018.02855. eCollection 2018. Front Immunol. 2018. PMID: 30619253 Free PMC article. - Thermal biology of mosquito-borne disease.
Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, Rohr JR, Ryan SJ, Savage V, Shocket MS, Sippy R, Stewart Ibarra AM, Thomas MB, Villena O. Mordecai EA, et al. Ecol Lett. 2019 Oct;22(10):1690-1708. doi: 10.1111/ele.13335. Epub 2019 Jul 8. Ecol Lett. 2019. PMID: 31286630 Free PMC article. Review. - Modelling the impact of climate change on the distribution and abundance of tsetse in Northern Zimbabwe.
Longbottom J, Caminade C, Gibson HS, Weiss DJ, Torr S, Lord JS. Longbottom J, et al. Parasit Vectors. 2020 Oct 19;13(1):526. doi: 10.1186/s13071-020-04398-3. Parasit Vectors. 2020. PMID: 33076987 Free PMC article.
References
- Colwell R. R. 1996. Global climate and infectious disease: the cholera paradigm. Science 274, 2025–203110.1126/science.274.5295.2025 (doi:10.1126/science.274.5295.2025) - DOI - DOI - PubMed
- Pascual M., Rodo X., Ellner S. P., Colwell R., Bouma M. J. 2000. Cholera dynamics and El Niño-southern oscillation. Science 289, 1766–176910.1126/science.289.5485.1766 (doi:10.1126/science.289.5485.1766) - DOI - DOI - PubMed
- Daszak P., Cunningham A. A., Hyatt A. D. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–44910.1126/science.287.5452.443 (doi:10.1126/science.287.5452.443) - DOI - DOI - PubMed
- Hay S. I., Cox J., Rogers D. J., Randolph S. E., Stern D. I., Shanks G. D., Myers M. F., Snow R. W. 2002. Climate change and the resurgence of malaria in the East African highlands. Nature 415, 905–90910.1038/415905a (doi:10.1038/415905a) - DOI - DOI - PMC - PubMed
- Pascual M., Ahumada J. A., Chaves L. F., Rodo X., Bouma M. J. 2006. Malaria resurgence in the East African highlands: temperature trends revisited. Proc. Natl Acad. Sci. USA. 103, 5829–583410.1073/pnas.0508929103 (doi:10.1073/pnas.0508929103) - DOI - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical