Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry - PubMed (original) (raw)
Review
Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry
Deepak P Srivastava et al. J Neurosci. 2011.
Abstract
Rapid actions of estrogens were first described >40 years ago. However, the importance of rapid estrogen-mediated actions in the CNS is only now becoming apparent. Several lines of evidence demonstrate that rapid estrogen-mediated signaling elicits potent effects on molecular and cellular events, resulting in the "fine-tuning" of neuronal circuitry. At an ultrastructural level, the details of estrogen receptor localization and how these are regulated by the circulating hormone and age are now becoming evident. Furthermore, the mechanisms that allow membrane-associated estrogen receptors to couple with intracellular signaling pathways are also now being revealed. Elucidation of complex actions of rapid estrogen-mediated signaling on synaptic proteins, connectivity, and synaptic function in pyramidal neurons has demonstrated that this neurosteroid engages specific mechanisms in different areas of the brain. The regulation of synaptic properties most likely underlies the fine-tuning of neuronal circuitry. This in turn may influence how learned behaviors are encoded by different circuitry in male and female subjects. Importantly, as estrogens have been suggested as potential treatments of a number of disorders of the CNS, advancements in our understanding of rapid estrogen signaling in the brain will serve to aid in the development of potential novel estrogen-based treatments.
Figures
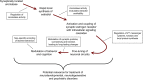
Figure 1.
Emerging evidence suggests that rapidly synthesized estradiol within the brain, mediated by synaptically located aromatase, is the source of rapid estrogen signaling in the brain. Importantly, the regulation of aromatase will modulate the availability of estradiol within the brain. Exposure of estradiol results in the activation of synaptically localized ERs. The functional coupling of ERs with G-protein-sensitive mechanisms results in the initiation of second messenger systems and allows for the activation of multiple intracellular cascades, including those which converge onto the actin cytoskeleton and regulation of local proteins synthesis. This in turns leads to the modulation of synaptic proteins, connectivity, and function. Alterations in synaptic properties result in long-term changes in neuronal circuitry. Ultimately, these changes in neuronal circuitry contribute to rapid estradiol influences in behavior and cognition. Moreover, such mechanisms influence the circuitry used to encode learned behaviors in a sex-specific manner.
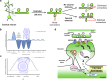
Figure 2.
Examples of rapid estrogen-induced effects on neuronal circuitry. a, Estradiol rapidly induces the formation of nascent synaptic connections in cortical neurons. Without a second stimulus, circuitry returns to control levels; addition of a second activity-dependent stimulus results in the stabilization and long-lasting increase in synaptic connectivity. b, c, The two diagrams illustrate persistent and opposite effects of acute stress on associative learning in females (b) versus males (c). Under unstressed conditions, females outperform males. However, after stress, females do not learn well, whereas males express more learned responses. The effects of stress on learning change numerous times across the female lifespan and can be ameliorated by various treatments and hormonal manipulations. In contrast, learning in males is less dynamic, with enhanced responding from puberty through middle age. d, The proposed model for rapid translational mechanism underlying estrogen-mediated synaptic plasticity in hippocampal neurons. Estrogen alleviates downstream translational repression, such as controlled by eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). This results in the upregulation of key synaptic modulators such as PSD-95 and GluA1, and may alter dendritic and synaptic structure as well as the facilitation of LTP.
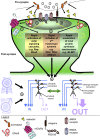
Figure 3.
Schematic of rapid estrogen actions in neurons. 1, Activation of synaptically located ERα or ERβ can result in the transactivation of mGluRs. 2, Activation of ER/mGluR (or just ER) results in the activation of specific intracellular mechanisms, including activation of kinases, small GTPases, or local protein synthesis mechanisms. Importantly, neurons in different areas of the brain (i.e., cortex vs hippocampus) result in the activation of distinct mechanisms. 3, Rapid activation of intracellular cascades induces changes in synaptic protein localization and expression levels. 4, The cumulative effect of rapid estrogen signaling results in the modulation of synaptic connectivity and synaptic function, and thus the fine-tuning of neuronal circuitry. 5, Estrogen-mediated fine-tuning of specific neuronal circuitry for the encoding of specific cognitive behaviors is regulated by both sex and stressful events.
Similar articles
- Synaptic effects of estrogen.
Nicholson K, MacLusky NJ, Leranth C. Nicholson K, et al. Vitam Horm. 2020;114:167-210. doi: 10.1016/bs.vh.2020.06.002. Epub 2020 Jul 20. Vitam Horm. 2020. PMID: 32723543 Review. - Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection.
Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Cardona-Gómez GP, et al. Brain Res Brain Res Rev. 2001 Nov;37(1-3):320-34. doi: 10.1016/s0165-0173(01)00137-0. Brain Res Brain Res Rev. 2001. PMID: 11744097 Review. - Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition.
Sellers K, Raval P, Srivastava DP. Sellers K, et al. Front Neuroendocrinol. 2015 Jan;36:72-89. doi: 10.1016/j.yfrne.2014.08.001. Epub 2014 Aug 23. Front Neuroendocrinol. 2015. PMID: 25159586 Review. - Membrane-initiated signaling of estrogen in the brain.
Rønnekleiv OK, Malyala A, Kelly MJ. Rønnekleiv OK, et al. Semin Reprod Med. 2007 May;25(3):165-77. doi: 10.1055/s-2007-973429. Semin Reprod Med. 2007. PMID: 17447206 Review. - Cooperation of Genomic and Rapid Nongenomic Actions of Estrogens in Synaptic Plasticity.
Lai YJ, Yu D, Zhang JH, Chen GJ. Lai YJ, et al. Mol Neurobiol. 2017 Aug;54(6):4113-4126. doi: 10.1007/s12035-016-9979-y. Epub 2016 Jun 20. Mol Neurobiol. 2017. PMID: 27324789 Free PMC article. Review.
Cited by
- 17⍺-Estradiol Protects against HIV-1 Tat-Induced Endolysosome Dysfunction and Dendritic Impairments in Neurons.
Datta G, Miller NM, Chen X. Datta G, et al. Cells. 2023 Mar 6;12(5):813. doi: 10.3390/cells12050813. Cells. 2023. PMID: 36899948 Free PMC article. - A computational lens on menopause-associated psychosis.
Fisher VL, Ortiz LS, Powers AR 3rd. Fisher VL, et al. Front Psychiatry. 2022 Aug 3;13:906796. doi: 10.3389/fpsyt.2022.906796. eCollection 2022. Front Psychiatry. 2022. PMID: 35990063 Free PMC article. - 17β-estradiol enhances memory duration in the main olfactory bulb in CD-1 mice.
Dillon TS, Fox LC, Han C, Linster C. Dillon TS, et al. Behav Neurosci. 2013 Dec;127(6):923-31. doi: 10.1037/a0034839. Behav Neurosci. 2013. PMID: 24341716 Free PMC article. - Epigenetic regulation of estrogen-dependent memory.
Fortress AM, Frick KM. Fortress AM, et al. Front Neuroendocrinol. 2014 Oct;35(4):530-49. doi: 10.1016/j.yfrne.2014.05.001. Epub 2014 May 28. Front Neuroendocrinol. 2014. PMID: 24878494 Free PMC article. Review. - Estrogen modulation of visceral nociceptors.
Chaban V. Chaban V. Curr Trends Neurol. 2013;7:51-55. Curr Trends Neurol. 2013. PMID: 26752851 Free PMC article.
References
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annu Rev Neurosci. 2007;30:79–97. - PubMed
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS051923/NS/NINDS NIH HHS/United States
- R21 AG039850/AG/NIA NIH HHS/United States
- R01 NS041302/NS/NINDS NIH HHS/United States
- MH059970/MH/NIMH NIH HHS/United States
- P30 NS062158/NS/NINDS NIH HHS/United States
- AG039850/AG/NIA NIH HHS/United States
- MH-59970/MH/NIMH NIH HHS/United States
- NS062158/NS/NINDS NIH HHS/United States
- AG167065/AG/NIA NIH HHS/United States
- NS045260/NS/NINDS NIH HHS/United States
- P01 NS045260/NS/NINDS NIH HHS/United States
- R01 MH059970/MH/NIMH NIH HHS/United States
- NS41302/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources