Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma - PubMed (original) (raw)
Review
Mechanism of action of proteasome inhibitors and deacetylase inhibitors and the biological basis of synergy in multiple myeloma
Teru Hideshima et al. Mol Cancer Ther. 2011 Nov.
Abstract
Novel agents, including the proteasome inhibitor bortezomib, have significantly improved the response and survival of patients with multiple myeloma over the last decade. Despite these advances, many patients relapse or do not benefit from the currently available therapies; thus, multiple myeloma remains an incurable disease. Deacetylase inhibitors (DACi), including panobinostat and vorinostat, have recently emerged as novel agents being evaluated in the treatment of multiple myeloma. Deacetylases are a group of enzymes with effects on various intracellular proteins, including histones, transcription factors, and molecular chaperones. Although DACi inhibit cell growth and induce apoptosis in multiple myeloma cells as a single agent, synergistic activity has been observed when they were used in combination with bortezomib. The mechanistic basis of synergy is multifactorial and includes disruption of protein degradation and inhibition of the interaction of multiple myeloma cells with the tumor microenvironment. This review summarizes recent advancements in the understanding of the mechanism of action of proteasome inhibitors and DACi in multiple myeloma and examines the biological basis of their synergistic effects. Data from the studies summarized here have been used as the rationale for the implementation of phase II and III clinical trials of DACi, alone and combined with bortezomib, in relapsed and refractory multiple myeloma.
Conflict of interest statement
Disclosure of Potential Conflict of Interest disclosure
T.H. is a consultant for Acetylon Pharmaceuticals. P.G.R. is a consultant and on advisory boards for Millennium and Celgene. K.C.A. is a consultant and on advisory board for Millennium, Celgene, and Novartis.
Figures
Figure 1
Molecular structures of deacetylase inhibitors and the proteasome inhibitor bortezomib.
Figure 2
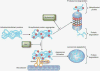
Inhibitions of the proteasome and aggresome pathways by bortezomib and deacetylase inhibitors (DACi). Unfolded/misfolded proteins are targeted by ubiquitin for degradation by the proteasome and aggresome pathways. The proteasome inhibitor bortezomib leads to the accumulation of ubiquitin protein aggregates. These aggregates are shuttled to the lysosome, where they are degraded via the aggresome pathway. Aggresome formation involves the shuttling of the protein aggregates along microtubules by dynein motor proteins. The interaction of the unfolded/misfolded protein complexes is facilitated by histone deacetylase 6 (HDAC6). Conversely, DACi with inhibitory activity toward HDAC6 block this process. The combination of proteasome inhibitors and DACi lead to increased cellular stress and apoptosis.
Figure 3
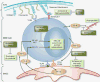
Bortezomib and deacetylase inhibitors (DACi) inhibit key pathways associated with multiple myeloma (MM) cell growth and survival. Growth and survival of MM cells are dependent on functioning intracellular pathways that drive proliferation of and the interaction with the extracellular matrix (ECM) and bone marrow stromal cells (BMSC). The combination of bortezomib and DACi leads to inactivation of NF-κB and MM cell apoptosis. Both DACi and bortezomib suppress the production of cytokines including interleukin-6 (IL-6) and insulin-like growth factor 1 (IGF1). Bortezomib also suppresses tumor necrosis factor α (TNF-α), leading to inhibition of the interaction of MM cells and BMSCs. Bortezomib has been shown to decrease the secretion of VEGF, leading to inhibition of angiogenesis. The cell surface proteoglycan syndecan 1 is downregulated by DACi, which affects the interaction of MM cells with the ECM. ICAM, intracellular adhesion molecule; LFA4, leukocyte function-associated antigen 4; STAT3, signal transducers and activators of transcription 3; VCAM, vascular cell adhesion molecule; VLA4, very late antigen 4.
Similar articles
- Combined proteasome and histone deacetylase inhibition: A promising synergy for patients with relapsed/refractory multiple myeloma.
Jagannath S, Dimopoulos MA, Lonial S. Jagannath S, et al. Leuk Res. 2010 Sep;34(9):1111-8. doi: 10.1016/j.leukres.2010.04.001. Epub 2010 May 15. Leuk Res. 2010. PMID: 20472288 Review. - Proteasome inhibitor therapy in multiple myeloma.
Chauhan D, Hideshima T, Mitsiades C, Richardson P, Anderson KC. Chauhan D, et al. Mol Cancer Ther. 2005 Apr;4(4):686-92. doi: 10.1158/1535-7163.MCT-04-0338. Mol Cancer Ther. 2005. PMID: 15827343 Review. - Discovery, Development, and clinical applications of bortezomib.
Jung L, Holle L, Dalton WS. Jung L, et al. Oncology (Williston Park). 2004 Dec;18(14 Suppl 11):4-13. Oncology (Williston Park). 2004. PMID: 15688597 Review. - Bortezomib: a valuable new antineoplastic strategy in multiple myeloma.
Bladé J, Cibeira MT, Rosiñol L. Bladé J, et al. Acta Oncol. 2005;44(5):440-8. doi: 10.1080/02841860510030002. Acta Oncol. 2005. PMID: 16118077 Review. - Proteasome inhibitor, bortezomib, for myeloma and lymphoma.
Tobinai K. Tobinai K. Int J Clin Oncol. 2007 Oct;12(5):318-26. doi: 10.1007/s10147-007-0695-5. Epub 2007 Oct 22. Int J Clin Oncol. 2007. PMID: 17929113 Review.
Cited by
- Roadmap for new practitioners to navigate the multiple myeloma landscape.
Tam T, Smith E, Lozoya E, Heers H, Andrew Allred P. Tam T, et al. Heliyon. 2022 Sep 12;8(9):e10586. doi: 10.1016/j.heliyon.2022.e10586. eCollection 2022 Sep. Heliyon. 2022. PMID: 36164513 Free PMC article. Review. - Discovery of selective small-molecule HDAC6 inhibitor for overcoming proteasome inhibitor resistance in multiple myeloma.
Hideshima T, Qi J, Paranal RM, Tang W, Greenberg E, West N, Colling ME, Estiu G, Mazitschek R, Perry JA, Ohguchi H, Cottini F, Mimura N, Görgün G, Tai YT, Richardson PG, Carrasco RD, Wiest O, Schreiber SL, Anderson KC, Bradner JE. Hideshima T, et al. Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):13162-13167. doi: 10.1073/pnas.1608067113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799547 Free PMC article. - CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma.
Murray ME, Gavile CM, Nair JR, Koorella C, Carlson LM, Buac D, Utley A, Chesi M, Bergsagel PL, Boise LH, Lee KP. Murray ME, et al. Blood. 2014 Jun 12;123(24):3770-9. doi: 10.1182/blood-2013-10-530964. Epub 2014 Apr 29. Blood. 2014. PMID: 24782505 Free PMC article. - Carfilzomib Triple Combination Therapy: A Review in Relapsed Multiple Myeloma.
Hoy SM. Hoy SM. Target Oncol. 2016 Apr;11(2):255-62. doi: 10.1007/s11523-016-0428-7. Target Oncol. 2016. PMID: 26972294 Review. - High-Throughput Drug Screening and Multi-Omic Analysis to Guide Individualized Treatment for Multiple Myeloma.
Coffey DG, Cowan AJ, DeGraaff B, Martins TJ, Curley N, Green DJ, Libby EN, Silbermann R, Chien S, Dai J, Morales A, Gooley TA, Warren EH, Becker PS. Coffey DG, et al. JCO Precis Oncol. 2021 Apr 6;5:PO.20.00442. doi: 10.1200/PO.20.00442. eCollection 2021. JCO Precis Oncol. 2021. PMID: 34250400 Free PMC article. Clinical Trial.
References
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
- Kaufman J, Gleason C, Lonial S. Treatment of relapsed and refractory myeloma. Curr Hematol Malig Rep. 2009;4:99–107. - PubMed
- Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–7. - PMC - PubMed
- San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–17. - PubMed
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P0-1 78378/PHS HHS/United States
- IP50 CA-10070/CA/NCI NIH HHS/United States
- P01 CA078378/CA/NCI NIH HHS/United States
- R01 CA050947/CA/NCI NIH HHS/United States
- P50 CA100707/CA/NCI NIH HHS/United States
- R0-1 CA-50947/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical