ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia - PubMed (original) (raw)
ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia
Benjamin Bondue et al. PLoS Pathog. 2011 Nov.
Abstract
Viral diseases of the respiratory tract, which include influenza pandemic, children acute bronchiolitis, and viral pneumonia of the elderly, represent major health problems. Plasmacytoid dendritic cells play an important role in anti-viral immunity, and these cells were recently shown to express ChemR23, the receptor for the chemoattractant protein chemerin, which is expressed by epithelial cells in the lung. Our aim was to determine the role played by the chemerin/ChemR23 system in the physiopathology of viral pneumonia, using the pneumonia virus of mice (PVM) as a model. Wild-type and ChemR23 knock-out mice were infected by PVM and followed for functional and inflammatory parameters. ChemR23(-/-) mice displayed higher mortality/morbidity, alteration of lung function, delayed viral clearance and increased neutrophilic infiltration. We demonstrated in these mice a lower recruitment of plasmacytoid dendritic cells and a reduction in type I interferon production. The role of plasmacytoid dendritic cells was further addressed by performing depletion and adoptive transfer experiments as well as by the generation of chimeric mice, demonstrating two opposite effects of the chemerin/ChemR23 system. First, the ChemR23-dependent recruitment of plasmacytoid dendritic cells contributes to adaptive immune responses and viral clearance, but also enhances the inflammatory response. Second, increased morbidity/mortality in ChemR23(-/-) mice is not due to defective plasmacytoid dendritic cells recruitment, but rather to the loss of an anti-inflammatory pathway involving ChemR23 expressed by non-leukocytic cells. The chemerin/ChemR23 system plays important roles in the physiopathology of viral pneumonia, and might therefore be considered as a therapeutic target for anti-viral and anti-inflammatory therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
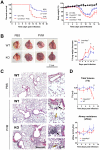
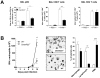
Figure 1. Higher mortality rate and more severe pathological features in ChemR23−/− mice infected by PVM.
(A) Following intranasal inoculation of PVM (1000 PFUs) or control medium, ChemR23−/− and wild-type (WT) C57BL/6 mice were monitored daily for survival (left panel) and weight loss (right panel). Weight curves (mean ± SEM) are relative to initial body weight. Differences were significant (p<0.05) between infected wild-type (blue curve) and ChemR23−/− (red curve) mice from day 7 to day 16. The displayed data result from the pooling of four independent experiments (n = 40 for infected groups and n = 10 for uninfected groups). (B) Macroscopic aspect of lungs collected from WT and ChemR23−/− (KO) mice 9 days after PVM infection or PBS instillation. The weight of collected lungs was assessed at various time-points post-infection (right panel) and expressed as percentage over lung weight of uninfected control mice. The data shown are the mean ± SEM for groups of seven animals. (C) Representative sections of lungs stained with hematoxylin and eosin. Scale bars: 500 µm for left panels, 50 µm for right panels. PVM-infected lungs display perivascular (black arrows), peribronchiolar (white arrow), and alveolar inflammation (yellow arrows). (D) Respiratory dysfunction was measured using a double chamber plethysmograph. Before and at selected time points after intranasal inoculation of PVM, tidal volume (TV) and specific airway resistance (sRaw) were determined in wild-type and ChemR23−/− mice. The data shown are the mean ± SEM for groups of four animals. All displayed data are representative of at least three independent experiments. *, p<0.05; **, p<0.01.
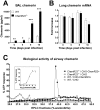
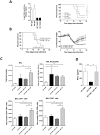
Figure 2. Increased chemerin levels in broncho-alveolar lavage fluid during PVM infection.
(A) At selected time points after viral inoculation, chemerin levels were determined by ELISA in BAL fluids of ChemR23−/− and wild-type C57BL/6 mice. (B) Chemerin transcript levels were determined by quantitative RT-PCR. The data were normalized using two housekeeping genes (YWHAZ and CANX) as references, and reported to the chemerin transcript level in uninfected control mice. The displayed data are the mean ± SEM for groups of minimum seven animals. (C) The biological activity of chemerin was measured in BAL fluids obtained at day 9 post-infection from wild-type (WT) and ChemR23−/− mice after a reverse phase HPLC fractionation (C18 column) using an aequorin-based intracellular Ca2+ mobilization assay. The data shown are the activity of fractions (25 to 50% acetonitrile) normalized to the response to ATP upon testing on CHO-K1 cells expressing mouse ChemR23 (CHO-ChemR23+) or not (CHO). The functional response of mouse ChemR23-expressing CHO-K1 cells to recombinant mouse chemerin is shown as inset. Data are representative of three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
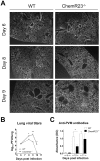
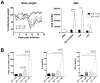
Figure 3. Increased viral load and delayed viral clearance in ChemR23−/− mice.
(A) Immunofluorescent staining of viral antigens in lung sections at 6, 8, and 9 days post-infection of wild-type (WT) and ChemR23−/− mice. Original magnification: ×50. (B) Viral titers were determined in lung homogenates for WT (open squares) and ChemR23−/− (filled triangles) mice at selected time points post-infection, and expressed in log10 plaque-forming units (PFU) per lung. (C) At days 9, 10, and 11 post-infection, the serum of infected mice was assessed for the presence of anti-PVM antibodies by ELISA. The displayed data are the mean ± SEM for groups of seven animals and are representative of at least two independent experiments. *, p<0.05; **, p<0.01.
Figure 4. Reduced levels of type I IFNs and IL-12p40 in ChemR23−/− infected mice.
At selected time points after infection, lungs and/or broncho-alveolar lavage fluids obtained from wild-type (WT) (white bars) and ChemR23−/− mice (black bars) were assessed for cytokine transcripts or proteins. (A) Lung IFN-α and IFN-β transcripts were assayed by qRT-PCR (upper panels). IFN-α levels were also determined by ELISA in lung homogenates (lower left panel) and broncho-alveolar lavage fluids (lower right panel). (B) Chemokine (KC/CXCL1) and cytokines (IFN-γ, TNF-α, IL-6, IL-12p40 and IL-17) levels were determined by ELISA in lung homogenates. Data are the mean ± SEM for groups of seven animals and are representative of three independent experiments. *, p<0.05; **, p<0.01.
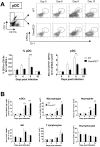
Figure 5. Plasmacytoid dendritic cell recruitment to the lung is less efficient in ChemR23−/− mice during PVM infection.
Inflammatory cell subsets were assessed by flow cytometry in lung cell suspensions obtained from wild-type (WT) (white bars) and ChemR23−/− mice (black bars) either naive or 6, 8, and 10 days after PVM inoculation. (A) Plasmacytoid dendritic cells (pDC) were determined as the Gr-1+ mPDCA+ population after gating on CD11b− CD11c+ cells (upper panels). The lower panels display respectively the percentage and absolute numbers of pDCs. (B) Absolute numbers of myeloid dendritic cells (mDC, CD11c+ CD11b+ MHC IIhigh F4-80− cells), lung macrophages (F4-80+ MHC IIlow CD11b+), neutrophils (Gr1+ CD11b+ CD11c−), NK cells (NK1-1+ CD3−), T (CD19− CD3+) and B (CD19+ CD3−) lymphocytes at selected time points after infection. Data are the mean ± SEM for groups of at least five animals and are representative of at least three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
Figure 6. Decreased recruitment of pDCs and CD8+ T lymphocytes and higher recruitment of neutrophils to the lung of infected ChemR23-deficient mice.
(A) Absolute numbers of pDCs, CD8+ and CD4+ T lymphocytes determined by flow cytometry in BAL fluids obtained from wild-type (WT) (white bars) and ChemR23−/− mice (black bars), either non-infected or at day 8 post-infection. (B) Neutrophil counts were determined in BAL fluids at different time points post-infection in WT and ChemR23−/− mice (left panel). Hematoxylin-eosin staining as well as the relative cell counts performed on cytospin preparations (middle and right panels) from BAL fluids obtained from wild-type (white bars) and ChemR23−/− mice (black bars) at day 10 post-infection are displayed. Data are the mean ± SEM for groups of at least five animals. *, p<0.05; **, p<0.01; ***, p<0.001.
Figure 7. Increased pathogenicity of PVM in ChemR23−/− mice is not due to the defective pDC recruitment.
(A) Depletion of pDCs was achieved by using the 120G8 monoclonal antibody in wild-type (WT) and ChemR23−/− (KO) mice infected by PVM. Depletion efficacy was assessed by flow cytometry analysis of pDCs (CD11b− CD11c+ B220+ mPDCA+) among spleen cells collected at day 8 post-infection (left panel). Survival was recorded for infected WT and ChemR23−/− mice, depleted or not in pDCs (n = 7 per group) (right panel). (B) 106 pDCs from WT or ChemR23−/− mice were transferred intravenously into knock-out mice at the time of infection with PVM. Knock-out mice receiving a saline solution were used as controls. Survival (left panel) and body weight (right panel) were monitored (n = 6 per group). (C) 106 pDCs from WT or ChemR23−/− mice were transferred intravenously into ChemR23−/− mice at the time of infection (n = 7 per group). Infected wild-type and knock-out mice receiving a saline solution were used as controls (n = 5 per group). Mice were sacrificed 14 days after infection and the cell populations were analyzed in BAL fluids using flow cytometry. Histograms show the mean ± SEM for total cell numbers and absolute numbers of neutrophils, CD3+CD4+ and CD3+CD8+ T lymphocytes. (D) 106 pDCs from WT mice were transferred intravenously into ChemR23−/− mice at the time of infection. Infected wild-type and knock-out mice were used as controls. IFN-α levels were detected 9 days after infection in BAL fluids using ELISA (n = 5 per group). *, p<0.05; **, p<0.01, ***, p<0.001.
Figure 8. Transfer of bone marrow from wild-type mice does not protect ChemR23-deficient mice from PVM infection.
(A) Irradiated (*) wild-type mice reconstituted with ChemR23−/− bone marrow (WT*+KO), as well as irradiated KO mice reconstituted with bone marrow from WT mice (KO*+WT), were infected by PVM (1000 PFUs) and monitored for weight loss (left panel). 23 days post-infection, mice were sacrificed and inflammatory cells were counted in BAL fluids (right panel). (B) BAL fluids were also harvested at day 14 post-infection in a similar experiment including two additional groups, namely WT and KO mice reconstituted respectively with WT and KO bone marrow (WT*+WT, KO*+KO, respectively). Total cell numbers (left panel) as well as cytokine levels (IL-6 and IFN-γ, middle and right panels) were determined in these samples. Data are the mean ± SEM for groups of at least five animals. *, p<0.05; **, p<0.01.
Similar articles
- Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model.
Luangsay S, Wittamer V, Bondue B, De Henau O, Rouger L, Brait M, Franssen JD, de Nadai P, Huaux F, Parmentier M. Luangsay S, et al. J Immunol. 2009 Nov 15;183(10):6489-99. doi: 10.4049/jimmunol.0901037. Epub 2009 Oct 19. J Immunol. 2009. PMID: 19841182 - Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice.
Vanderstocken G, Van de Paar E, Robaye B, di Pietrantonio L, Bondue B, Boeynaems JM, Desmecht D, Communi D. Vanderstocken G, et al. PLoS One. 2012;7(11):e50385. doi: 10.1371/journal.pone.0050385. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185614 Free PMC article. - Chemerin peptides promote phagocytosis in a ChemR23- and Syk-dependent manner.
Cash JL, Christian AR, Greaves DR. Cash JL, et al. J Immunol. 2010 May 1;184(9):5315-24. doi: 10.4049/jimmunol.0903378. Epub 2010 Apr 2. J Immunol. 2010. PMID: 20363975 Free PMC article. - Chemerin/chemR23 axis in inflammation onset and resolution.
Mariani F, Roncucci L. Mariani F, et al. Inflamm Res. 2015 Feb;64(2):85-95. doi: 10.1007/s00011-014-0792-7. Epub 2014 Dec 30. Inflamm Res. 2015. PMID: 25548799 Review. - Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism.
Bondue B, Wittamer V, Parmentier M. Bondue B, et al. Cytokine Growth Factor Rev. 2011 Oct-Dec;22(5-6):331-8. doi: 10.1016/j.cytogfr.2011.11.004. Epub 2011 Nov 25. Cytokine Growth Factor Rev. 2011. PMID: 22119008 Review.
Cited by
- Dynamic and tissue-specific proteolytic processing of chemerin in obese mice.
Zhao L, Yamaguchi Y, Shen WJ, Morser J, Leung LLK. Zhao L, et al. PLoS One. 2018 Aug 30;13(8):e0202780. doi: 10.1371/journal.pone.0202780. eCollection 2018. PLoS One. 2018. PMID: 30161155 Free PMC article. - Contrasting Effects of Adipokines on the Cytokine Production by Primary Human Bronchial Epithelial Cells: Inhibitory Effects of Adiponectin.
Salvator H, Grassin-Delyle S, Naline E, Brollo M, Fournier C, Couderc LJ, Devillier P. Salvator H, et al. Front Pharmacol. 2020 Feb 18;11:56. doi: 10.3389/fphar.2020.00056. eCollection 2020. Front Pharmacol. 2020. PMID: 32132922 Free PMC article. - Molecular imaging of chemokine-like receptor 1 (CMKLR1) in experimental acute lung injury.
Mannes PZ, Barnes CE, Biermann J, Latoche JD, Day KE, Zhu Q, Tabary M, Xiong Z, Nedrow JR, Izar B, Anderson CJ, Villanueva FS, Lee JS, Tavakoli S. Mannes PZ, et al. Proc Natl Acad Sci U S A. 2023 Jan 17;120(3):e2216458120. doi: 10.1073/pnas.2216458120. Epub 2023 Jan 10. Proc Natl Acad Sci U S A. 2023. PMID: 36626557 Free PMC article. - Chemerin partly mediates tumor-inhibitory effect of all-trans retinoic acid via CMKLR1-dependent natural killer cell recruitment.
Song Y, Yin W, Dan Y, Sheng J, Zeng Y, He R. Song Y, et al. Immunology. 2019 Jul;157(3):248-256. doi: 10.1111/imm.13065. Epub 2019 May 29. Immunology. 2019. PMID: 31063220 Free PMC article. - CMKLR1 activation ex vivo does not increase proportionally to serum total chemerin in obese humans.
Toulany J, Parlee SD, Sinal CJ, Slayter K, McNeil S, Goralski KB. Toulany J, et al. Endocr Connect. 2016 Nov;5(6):70-81. doi: 10.1530/EC-16-0065. Epub 2016 Nov 8. Endocr Connect. 2016. PMID: 27881447 Free PMC article.
References
- Simoes EA. Respiratory syncytial virus infection. Lancet. 1999;354:847–852. - PubMed
- Howard TS, Hoffman LH, Stang PE, Simoes EA. Respiratory syncytial virus pneumonia in the hospital setting: length of stay, charges, and mortality. J Pediatr. 2000;137:227–232. - PubMed
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. - PubMed
- Hutchinson AF, Ghimire AK, Thompson MA, Black JF, Brand CA, et al. A community-based, time-matched, case-control study of respiratory viruses and exacerbations of COPD. Respir Med. 2007;101:2472–2481. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases