Laser-based single-axon transection for high-content axon injury and regeneration studies - PubMed (original) (raw)
Laser-based single-axon transection for high-content axon injury and regeneration studies
Darío Kunik et al. PLoS One. 2011.
Abstract
The investigation of the regenerative response of the neurons to axonal injury is essential to the development of new axoprotective therapies. Here we study the retinal neuronal RGC-5 cell line after laser transection, demonstrating that the ability of these cells to initiate a regenerative response correlates with axon length and cell motility after injury. We show that low energy picosecond laser pulses can achieve transection of unlabeled single axons in vitro and precisely induce damage with micron precision. We established the conditions to achieve axon transection, and characterized RGC-5 axon regeneration and cell body response using time-lapse microscopy. We developed an algorithm to analyze cell trajectories and established correlations between cell motility after injury, axon length, and the initiation of the regeneration response. The characterization of the motile response of axotomized RGC-5 cells showed that cells that were capable of repair or regrowth of damaged axons migrated more slowly than cells that could not. Moreover, we established that RGC-5 cells with long axons could not recover their injured axons, and such cells were much more motile. The platform we describe allows highly controlled axonal damage with subcellular resolution and the performance of high-content screening in cell cultures.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
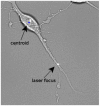
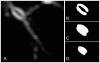
Figure 1. Axon transected with a focused picosecond laser beam.
The pulse energy was 7.5 nJ at 76 MHz, with an exposure of 5 sec.
Figure 2. Longitudinal changes in axonal morphology after laser axotomy.
A. Cell before injury and a magnification of the axon shown in an inset. B. After laser exposure there was swelling of the distal axon adjacent to the injury site. C. 20 min after exposure an approximately 1 m separation became apparent between the ends of the axon proximal and distal to the injury site. D. The loss of membrane integrity was evidenced by bubbles and distal axon fragmentation. Scale bars: 10  m. (See Supplemental Video S1 for the full series).
m. (See Supplemental Video S1 for the full series).
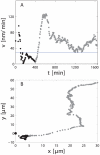
Figure 3. Soma motility response to axonal injury quantitative analysis.
A. Instantaneous velocity and B. trajectory of the cell shown in Supplemental Video S4. Transection occurred at t = 0. The horizontal line in A shows the mean baseline velocity of control cells. Point were acquired every 5 minutes approximatelly.
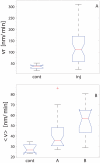
Figure 4. Soma motility response to axonal injury group comparisons.
A. Initial reaction and B. long-term response. Cell motility changes were correlated to the regenerative response. Cell group labels are inj for injured cells, cont for control cells, A for that cells that either regrowth new axons or repaired injured one and, B represents cells that were not able either to regenerate or replace injured axons.
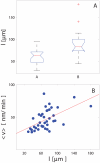
Figure 5. Effects of axon length on regeneration and motility.
A. The regenerative response of cells depended on axon length. Cells with longer axons were less likely to reconnect or regrow their axons after axotomy. B. Correlation between cell motility and axon length. Cell with longer axons showed a higher motility after axotomy (R = 0.3 for linear regression).
Figure 6. Cell motility after injury depends on whether an axon regeneration program is initiated.
Somas that regenerated or repaired injured axons were more likely to migrate towards the site of axon transection (A), while somas that did not maintain their axons migrated away from the transection site (B).
Figure 7. Intermediate steps in the image analysis algorithm.
A. Standard deviation of the image intensity over a 90-pixel matrix. B. Thresholding. C. Opening and filling morphological operations. D. Erosion yielding the final mask.
Similar articles
- Applications of Proteomics to Nerve Regeneration Research.
Massing MW, Robinson GA, Marx CE, Alzate O, Madison RD. Massing MW, et al. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review. - Regenerative Responses and Axon Pathfinding of Retinal Ganglion Cells in Chronically Injured Mice.
Yungher BJ, Ribeiro M, Park KK. Yungher BJ, et al. Invest Ophthalmol Vis Sci. 2017 Mar 1;58(3):1743-1750. doi: 10.1167/iovs.16-19873. Invest Ophthalmol Vis Sci. 2017. PMID: 28324115 Free PMC article. - Promoting axon regeneration by inhibiting RNA N6-methyladenosine demethylase ALKBH5.
Wang D, Zheng T, Zhou S, Liu M, Liu Y, Gu X, Mao S, Yu B. Wang D, et al. Elife. 2023 Aug 3;12:e85309. doi: 10.7554/eLife.85309. Elife. 2023. PMID: 37535403 Free PMC article. - Postinjury Induction of Activated ErbB2 Selectively Hyperactivates Denervated Schwann Cells and Promotes Robust Dorsal Root Axon Regeneration.
Han SB, Kim H, Lee H, Grove M, Smith GM, Son YJ. Han SB, et al. J Neurosci. 2017 Nov 8;37(45):10955-10970. doi: 10.1523/JNEUROSCI.0903-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982707 Free PMC article. - Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge.
Min Q, Parkinson DB, Dun XP. Min Q, et al. Glia. 2021 Feb;69(2):235-254. doi: 10.1002/glia.23892. Epub 2020 Jul 22. Glia. 2021. PMID: 32697392 Review.
Cited by
- A Brief Review of In Vitro Models for Injury and Regeneration in the Peripheral Nervous System.
Varier P, Raju G, Madhusudanan P, Jerard C, Shankarappa SA. Varier P, et al. Int J Mol Sci. 2022 Jan 13;23(2):816. doi: 10.3390/ijms23020816. Int J Mol Sci. 2022. PMID: 35055003 Free PMC article. Review. - Tension- and Adhesion-Regulated Retraction of Injured Axons.
Shao X, You R, Hui TH, Fang C, Gong Z, Yan Z, Chang RCC, Shenoy VB, Lin Y. Shao X, et al. Biophys J. 2019 Jul 23;117(2):193-202. doi: 10.1016/j.bpj.2019.06.011. Epub 2019 Jun 20. Biophys J. 2019. PMID: 31278003 Free PMC article. - Network analysis of microRNAs, transcription factors, and target genes involved in axon regeneration.
Su LN, Song XQ, Xue ZX, Zheng CQ, Yin HF, Wei HP. Su LN, et al. J Zhejiang Univ Sci B. 2018 Apr.;19(4):293-304. doi: 10.1631/jzus.B1700179. J Zhejiang Univ Sci B. 2018. PMID: 29616505 Free PMC article.
References
- Raff MC, Whitmore AV, Finn JT. Axonal Self-Destruction and Neurodegeneration. Science. 2002;296:868–871. - PubMed
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–898. - PubMed
- Hilliard MA. Axonal degeneration and regeneration: a mechanistic tug-of-war. Journal of Neurochemistry. 2009;108:23–32. - PubMed
- Schwartz M, Yoles E, Levin LA. ‘axogenic’ and ‘somagenic’ neurodegenerative diseases: definitions and therapeutic implications. Molecular Medicine Today. 1999;5:470–473. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources