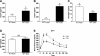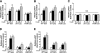Reproductive tissues maintain insulin sensitivity in diet-induced obesity - PubMed (original) (raw)
Comparative Study
Reproductive tissues maintain insulin sensitivity in diet-induced obesity
Sheng Wu et al. Diabetes. 2012 Jan.
Abstract
Reproductive dysfunction is associated with obesity. We previously showed that female mice with diet-induced obesity (DIO) exhibit infertility and thus serve as a model of human polycystic ovary syndrome (PCOS). We postulated that differential insulin signaling of tissues leads to reproductive dysfunction; therefore, a comparison of insulin signaling in reproductive tissues and energy storage tissues was performed. Pituitary-specific insulin receptor knockout mice were used as controls. High-fat diet-induced stress, which leads to insulin resistance, was also investigated by assaying macrophage infiltration and phosphorylated Jun NH(2)-terminal kinase (pJNK) signaling. In lean mice, reproductive tissues exhibited reduced sensitivity to insulin compared with peripheral metabolic tissues. However, in obese mice, where metabolic tissues exhibited insulin resistance, the pituitary and ovary maintained insulin sensitivity. Pituitaries responded to insulin through insulin receptor substrate (IRS)2 but not IRS1, whereas in the ovary, both IRS1 and IRS2 were activated by insulin. Macrophage infiltration and pJNK signaling were not increased in the pituitary or ovary of lean mice relative to DIO mice. The lack of inflammation and cytokine signaling in the pituitary and ovary in DIO mice compared with lean mice may be one of the reasons that these tissues remained insulin sensitive. Retained sensitivity of the pituitary and ovary to insulin may contribute to the pathophysiology of PCOS.
Figures
FIG. 1.
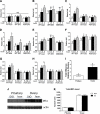
WT lean and DIO female mice were fasted overnight, and biochemical data are shown. A: Insulin. B: Leptin. C: Glucagon. D: IGF-I. E: Glucose tolerance test (2 g/kg i.p.
d
-glucose injected; glucose levels measured at different time points). n = 4–6/group. *,a,bSignificant differences (P < 0.05). NS, nonsignificant. GTT, glucose tolerance test.
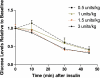
FIG. 2.
Time course of response to different doses of insulin in overnight-fasted lean female mice. Glucose was measured at 0, 10, 30, and 45 min. No significant decline in glucose levels was observed at any dose at 10 min. (A high-quality color representation of this figure is available in the online issue.)
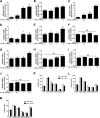
FIG. 3.
Fasted lean female mice were injected with saline or 0.5, 1, or 1.5 units/kg body wt insulin, and total AKT, pAKT, and pERK1/2 levels were measured in tissue homogenates using the Luminex analyzer. pAKT and pERK values are displayed relative to those of saline-injected mice and are corrected for total AKT. Total AKT values are expressed as relative light units. Different letters indicate significant differences between groups (P < 0.05). A and B: Liver. C and D: Muscle. E and F: Pituitary. G and H: Ovary. I and J: Hypothalamus. K: 0.5 units–total AKT. L: 1 unit–total AKT. M: 1.5 units–total AKT. NS, nonsignificant. pit, pituitary. hypo, hypothalamus.
FIG. 4.
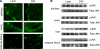
Western blot identifies differences in insulin-signaling pathways between reproductive tissues and the energy storage tissues. A: Female lean mice were fasted overnight and injected with insulin (1.5 units/kg body wt). B: pAKT levels of lean and DIO female mice were compared before or after insulin stimulation. (A high-quality color representation of this figure is available in the online issue.)
FIG. 5.
Luminex analysis of pAKT signaling after overnight-fasted mice were injected with insulin. WT lean, WT DIO, and PITIRKO-DIO mice were compared with regard to reproductive and energy storage tissues. n = 4–8/group. Bars with different letters are significantly different (P < 0.05). A: Pituitary. B: Ovary. C: Hypothalamus. D: Liver. E: Muscle.
FIG. 6.
Analysis of pTyr-IRS1 and pTyr-IRS2 signaling and IRS1 expression levels. A_–_H: pTyr-IRS1 (A_–_D) and pTyr-IRS2 (E_–_H) values of insulin-injected fasted mice are displayed relative to those of saline-injected mice. WT lean, WT DIO, and PITIRKO-DIO mice were compared with regard to reproductive and energy storage tissues. n = 4–8/group. A and E: Pituitary. B and F: Ovary. C and G: Liver. D and H: Muscle. I: irs1 mRNA level was measured in pituitary and ovary by real-time PCR in fed mice. J: IRS1 protein level shown by Western blot. K: IRS1 basal protein level was measured by Luminex assay in fed mice. Bars with different letters are significantly different (P < 0.05). (A high-quality color representation of this figure is available in the online issue.)
FIG. 7.
HFD-induced inflammation in different tissues. A: Macrophage infiltration was detected by immunohistochemistry using antibody F4/80. B: pJNK was examined by Western blot in different tissues. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 8.
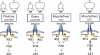
A model summarizing the insulin-signaling pathways in the liver, muscle, and pituitary of lean or DIO mice. Arrows indicate active signaling pathways, and an X over the arrow indicates blocked signaling pathways. PI3K, PI 3-kinase.
Similar articles
- Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor.
Brothers KJ, Wu S, DiVall SA, Messmer MR, Kahn CR, Miller RS, Radovick S, Wondisford FE, Wolfe A. Brothers KJ, et al. Cell Metab. 2010 Sep 8;12(3):295-305. doi: 10.1016/j.cmet.2010.06.010. Cell Metab. 2010. PMID: 20816095 Free PMC article. - Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell.
Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, Wolfe A. Wu S, et al. Diabetes. 2014 Apr;63(4):1270-82. doi: 10.2337/db13-1514. Epub 2013 Dec 30. Diabetes. 2014. PMID: 24379345 Free PMC article. - Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice.
DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A. DiVall SA, et al. PLoS One. 2015 Mar 17;10(3):e0119995. doi: 10.1371/journal.pone.0119995. eCollection 2015. PLoS One. 2015. PMID: 25780937 Free PMC article. - Advanced glycation end products and their relevance in female reproduction.
Merhi Z. Merhi Z. Hum Reprod. 2014 Jan;29(1):135-45. doi: 10.1093/humrep/det383. Epub 2013 Oct 30. Hum Reprod. 2014. PMID: 24173721 Review.
Cited by
- Low-Dose Dihydrotestosterone Drives Metabolic Dysfunction via Cytosolic and Nuclear Hepatic Androgen Receptor Mechanisms.
Andrisse S, Childress S, Ma Y, Billings K, Chen Y, Xue P, Stewart A, Sonko ML, Wolfe A, Wu S. Andrisse S, et al. Endocrinology. 2017 Mar 1;158(3):531-544. doi: 10.1210/en.2016-1553. Endocrinology. 2017. PMID: 27967242 Free PMC article. - AMP-activated protein kinase is a key intermediary in GnRH-stimulated LHβ gene transcription.
Andrade J, Quinn J, Becker RZ, Shupnik MA. Andrade J, et al. Mol Endocrinol. 2013 May;27(5):828-39. doi: 10.1210/me.2012-1323. Epub 2013 Mar 21. Mol Endocrinol. 2013. PMID: 23518923 Free PMC article. - The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1).
Wolfe A, Divall S, Wu S. Wolfe A, et al. Front Neuroendocrinol. 2014 Oct;35(4):558-72. doi: 10.1016/j.yfrne.2014.05.007. Epub 2014 Jun 12. Front Neuroendocrinol. 2014. PMID: 24929098 Free PMC article. Review. - Effect of High Fat Diet on Disease Development of Polycystic Ovary Syndrome and Lifestyle Intervention Strategies.
Han Y, Wu H, Sun S, Zhao R, Deng Y, Zeng S, Chen J. Han Y, et al. Nutrients. 2023 May 8;15(9):2230. doi: 10.3390/nu15092230. Nutrients. 2023. PMID: 37432488 Free PMC article. Review. - Polycystic Ovary Syndrome: Pathophysiology, Presentation, and Treatment With Emphasis on Adolescent Girls.
Witchel SF, Oberfield SE, Peña AS. Witchel SF, et al. J Endocr Soc. 2019 Jun 14;3(8):1545-1573. doi: 10.1210/js.2019-00078. eCollection 2019 Aug 1. J Endocr Soc. 2019. PMID: 31384717 Free PMC article. Review.
References
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med 2005;352:1223–1236 - PubMed
- Burks DJ, Font de Mora J, Schubert M, et al. IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 2000;407:377–382 - PubMed
- Murata Y, Tsuruzoe K, Kawashima J, et al. IRS-1 transgenic mice show increased epididymal fat mass and insulin resistance. Biochem Biophys Res Commun 2007;364:301–307 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK079637/DK/NIDDK NIH HHS/United States
- P60 DK079637/DK/NIDDK NIH HHS/United States
- U54 HD41859/HD/NICHD NIH HHS/United States
- R01 HD44608/HD/NICHD NIH HHS/United States
- R01 HD044608/HD/NICHD NIH HHS/United States
- U54 HD041859/HD/NICHD NIH HHS/United States
- P60DK079637/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous