Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury - PubMed (original) (raw)
Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury
Min D Tang-Schomer et al. Exp Neurol. 2012 Jan.
Abstract
Due to their viscoelastic nature, white matter axons are susceptible to damage by high strain rates produced during traumatic brain injury (TBI). Indeed, diffuse axonal injury (DAI) is one of the most common features of TBI, characterized by the hallmark pathological profiles of axonal bulbs at disconnected terminal ends of axons and periodic swellings along axons, known as "varicosities." Although transport interruption underlies axonal bulb formation, it is unclear how varicosities arise, with multiple sites accumulating transported materials along one axon. Recently, axonal microtubules have been found to physically break during dynamic stretch injury of cortical axons in vitro. Here, the same in vitro model was used in parallel with histopathological analyses of human brains acquired acutely following TBI to examine the potential role of mechanical microtubule damage in varicosity formation post-trauma. Transmission electron microscopy (TEM) following in vitro stretch injury revealed periodic breaks of individual microtubules along axons that regionally corresponded with undulations in axon morphology. However, typically less than a third of microtubules were broken in any region of an axon. Within hours, these sites of microtubule breaks evolved into periodic swellings. This suggests axonal transport may be halted along one broken microtubule, yet can proceed through the same region via other intact microtubules. Similar axonal undulations and varicosities were observed following TBI in humans, suggesting primary microtubule failure may also be a feature of DAI. These data indicate a novel mechanism of mechanical microtubule damage leading to partial transport interruption and varicosity formation in traumatic axonal injury.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
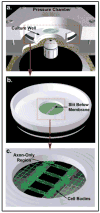
Figure 1. In vitro Model: Dynamic Stretch Injury of Axons
Pulsed air pressure introduces uniaxial stretch on patterned longitudinally arranged axon tracts, simulating stretch injury of CNS axons during head trauma. (a) The injury device consists of a culture well sealed within a pressure chamber into which a controlled air pulse is delivered. The culture well contains a central deformable membrane onto which primary cortical neuronal cells are plated (b). Two separate populations of cells are separated by a lithography fabricated micro-patterned barrier. Axonal processes extend through the 2mm microchannels to integrate with the opposing population of neurons, thus creating a unique axon-only region that can be placed over the slit in the device (c). Upon controlled delivery of air into the sealed chamber, the subsequent pressure change within the chamber allows the regionally specific deflection of the axon-only region.
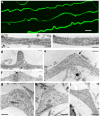
Figure 2. Microtubule Displacement and Undulation Formation Immediately After Dynamic Stretch Injury of Axons
(a) Representative images displaying multiple axons with an undulating morphology and immunoreactive for βIII-tubulin immediately (≤2 minutes) following dynamic stretch injury (Scale bar approx. 5μm). (b-c) TEM images showing a normal region of axon with intact microtubules aligned in parallel cables. (d-i) TEM images within 2 minutes following dynamic stretch injury showing widening of the spaces between microtubules near the peak of axon undulations accompanied by compaction of the polymers. Microtubules are also no longer linear and aligned in parallel but rather appear distorted and twisted with discrete breaks (arrows). Breaking and twisting of microtubules was observed near the peak of axon undulations where newly generated free-ends at disconnection points failed to align. Scale bars: 500 nm.
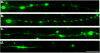
Figure 3. Immunocytochemical Staining of Axons Following Dynamic Stretch Injury
Immunofluorescent images of injured axons (3hr post-injury) displaying a series of swellings along the axonal length like beads on a string. Swellings display accumulations of (a) tubulin, (b) the microtubule-associated protein tau, (c) amyloid precursor protein (APP), and (d) neurofilament (NF200). Scale bar, 10 μm.
Figure 4. Microtubule Breakage and Loss in Varicose Axonal Swellings 3 Hours After Dynamic Stretch Injury of Axons
(a-d) TEM images of axons at 3hr post-injury were stitched together to reconstruct a panoramic image of an axon segment. Individual microtubules display selective breakage within axon swellings (a,c,d arrows). While breakage or loss of microtubules can be seen in association with swelling, other microtubules can be observed traversing the swollen region intact. Specifically, (b) shows a solitary intact microtubule traversing a large swelling (arrow) whereas multiple microtubules can be observed in the adjacent non-swollen region from which it emerged (arrow). This sole remaining microtubule feeds into a subsequent but separate swelling. Scale bars: 500 nm.
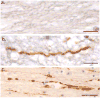
Figure 5. Undulations and Varicosities Following Acute Traumatic Brain Injury in Humans at Post-Mortem
(a) An absence of APP immunoreactivity in normal white matter within the corpus callosum of an 18F with no history of TBI who died as a result of haematological malignancy (case #5). Scale bar: 25μm (b) APP Immunoreactivity within the corpus callosum of an 18M who died 10 hours following acute severe traumatic brain injury caused by an assault (blunt force trauma to the head) (case #1). Axons display an undulating morphology akin to what is observed in vitro following dynamic stretch injury. Scale bar: 25μm (c) APP Immunoreactivity within the corpus callosum, also in case #1, displaying a classic varicose morphology with multiple individual swellings along the length of an individual axon can be observed. Scale bar: 30μm.
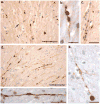
Figure 6. Varicose Axons within the Corpus Callosum of 4 Human Cases of Acute Severe TBI Examined at Post-Mortem via APP Immunohistochemistry
(a) Extensive axonal pathology with classic varicosities and axonal bulb formation within the corpus callosum of an 18F who died 22 hours following a MVC (case #3). Axons with an undulating morphology can be observed within the same field (arrow). Scale bar: 100μm (b and c) High magnification axonal varicosities in the corpus callosum of a 41 year-old male who died 16 hours following a fall (case #2). Note the serial swellings indicating multiple points of partial transport interruption. Scale bars (b):100μm, (c)30μm. (d) Extensive axonal pathology with classic varicosities and axonal bulb formation within the corpus callosum of an 18M who died 10 hours following blunt force trauma to the head (case #1). Scale bar: 100μm (e) High magnification of a single axon accumulating APP in a 25 year old male following a MVC (case #4). Note the multiple points of swelling along the visible axon length culminating in a large axonal swelling (axonal bulb) at the disconnected axon terminal. Scale bar: 90μm (f) Individual axon displaying a varicose morphology with isolated serial swellings, aslso from case #3. Scale bar: 50μm
Figure 7. Proposed Mechanism of Varicosity Formation after Traumatic Axonal Injury
(a) Illustration displaying two illustrated microtubules (MT1 and MT2) within an intact axon (pre-injury). (b) Following injury, mechanical breaking occurs at different sites in both microtubule 1 and microtubule 2. Misalignment of broken microtubules causes deformation of the axon observed as two discrete undulations. (c) Shortly afterward, catastrophic depolymerization from the broken ends of the microtubules allows the undulations to collapse and the axon recovers its linear morphology. (d) Microtubule breakage leads to impairment of axonal transport and subsequent accumulation of transported cargos near the microtubule breaking point. By contrast, axon transport on the intact microtubules remains normal. This ‘partial transport impairment’ may account for the formation of serial swellings that give axons a varicose appearance following traumatic axonal injury.
Similar articles
- CLARITY reveals a more protracted temporal course of axon swelling and disconnection than previously described following traumatic brain injury.
Weber MT, Arena JD, Xiao R, Wolf JA, Johnson VE. Weber MT, et al. Brain Pathol. 2019 May;29(3):437-450. doi: 10.1111/bpa.12677. Epub 2018 Dec 27. Brain Pathol. 2019. PMID: 30444552 Free PMC article. - SNTF immunostaining reveals previously undetected axonal pathology in traumatic brain injury.
Johnson VE, Stewart W, Weber MT, Cullen DK, Siman R, Smith DH. Johnson VE, et al. Acta Neuropathol. 2016 Jan;131(1):115-35. doi: 10.1007/s00401-015-1506-0. Epub 2015 Nov 20. Acta Neuropathol. 2016. PMID: 26589592 Free PMC article. - Mechanical breaking of microtubules in axons during dynamic stretch injury underlies delayed elasticity, microtubule disassembly, and axon degeneration.
Tang-Schomer MD, Patel AR, Baas PW, Smith DH. Tang-Schomer MD, et al. FASEB J. 2010 May;24(5):1401-10. doi: 10.1096/fj.09-142844. Epub 2009 Dec 17. FASEB J. 2010. PMID: 20019243 Free PMC article. - Axonal pathology in traumatic brain injury.
Johnson VE, Stewart W, Smith DH. Johnson VE, et al. Exp Neurol. 2013 Aug;246:35-43. doi: 10.1016/j.expneurol.2012.01.013. Epub 2012 Jan 20. Exp Neurol. 2013. PMID: 22285252 Free PMC article. Review. - Diffuse axonal injury in head trauma.
Smith DH, Meaney DF, Shull WH. Smith DH, et al. J Head Trauma Rehabil. 2003 Jul-Aug;18(4):307-16. doi: 10.1097/00001199-200307000-00003. J Head Trauma Rehabil. 2003. PMID: 16222127 Review.
Cited by
- Damage and Failure of Axonal Microtubule under Extreme High Strain Rate: An In-Silico Molecular Dynamics Study.
Wu YT, Adnan A. Wu YT, et al. Sci Rep. 2018 Aug 16;8(1):12260. doi: 10.1038/s41598-018-29804-w. Sci Rep. 2018. PMID: 30115936 Free PMC article. - Utilizing a Structural Mechanics Approach to Assess the Primary Effects of Injury Loads Onto the Axon and Its Components.
Montanino A, Kleiven S. Montanino A, et al. Front Neurol. 2018 Aug 6;9:643. doi: 10.3389/fneur.2018.00643. eCollection 2018. Front Neurol. 2018. PMID: 30127763 Free PMC article. - The Emergence of Model Systems to Investigate the Link Between Traumatic Brain Injury and Alzheimer's Disease.
Srinivasan G, Brafman DA. Srinivasan G, et al. Front Aging Neurosci. 2022 Feb 8;13:813544. doi: 10.3389/fnagi.2021.813544. eCollection 2021. Front Aging Neurosci. 2022. PMID: 35211003 Free PMC article. Review. - A high-efficiency model indicating the role of inhibition in the resilience of neuronal networks to damage resulting from traumatic injury.
Frost BL, Mintchev SM. Frost BL, et al. J Comput Neurosci. 2023 Nov;51(4):463-474. doi: 10.1007/s10827-023-00860-0. Epub 2023 Aug 26. J Comput Neurosci. 2023. PMID: 37632630 - Cerebrospinal fluid levels of neuroinflammatory biomarkers are increased in athletes with persistent post-concussive symptoms following sports-related concussion.
Gard A, Vedung F, Piehl F, Khademi M, Wernersson MP, Rorsman I, Tegner Y, Pessah-Rasmussen H, Ruscher K, Marklund N. Gard A, et al. J Neuroinflammation. 2023 Aug 17;20(1):189. doi: 10.1186/s12974-023-02864-0. J Neuroinflammation. 2023. PMID: 37592277 Free PMC article.
References
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. - PubMed
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12:565–572. - PubMed
- Faul M, Xu L, Wald MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta (GA): 2010.
- Galbraith JA, Thibault LE, Matteson DR. Mechanical and electrical responses of the squid giant axon to simple elongation. J Biomech Eng. 1993;115:13–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS056202/NS/NINDS NIH HHS/United States
- NS056202/NS/NINDS NIH HHS/United States
- R01 NS048949/NS/NINDS NIH HHS/United States
- R01 NS038104/NS/NINDS NIH HHS/United States
- R03 AG038911/AG/NIA NIH HHS/United States
- NS038104/NS/NINDS NIH HHS/United States
- NS048949/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources