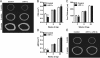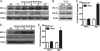Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation - PubMed (original) (raw)
Hypoxia-inducible factor-1α protein negatively regulates load-induced bone formation
Ryan C Riddle et al. J Biol Chem. 2011.
Abstract
Mechanical loads induce profound anabolic effects in the skeleton, but the molecular mechanisms that transduce such signals are still poorly understood. In this study, we demonstrate that the hypoxia-inducible factor-1α (Hif-1α) is acutely up-regulated in response to exogenous mechanical stimuli secondary to prostanoid signaling and Akt/mTOR (mammalian target of rapamycin) activation. In this context, Hif-1α associates with β-catenin to inhibit Wnt target genes associated with bone anabolic activity. Mice lacking Hif-1α in osteoblasts and osteocytes form more bone when subjected to tibia loading as a result of increased osteoblast activity. Taken together, these studies indicate that Hif-1α serves as a negative regulator of skeletal mechanotransduction to suppress load-induced bone formation by altering the sensitivity of osteoblasts and osteocytes to mechanical signals.
Figures
FIGURE 1.
Mice lacking Hif-1α in osteoblasts acquire increased cortical bone. A, representative microCT images illustrate cortical bone structure at the femoral mid-diaphysis in control and ΔHif-1α mice at 3, 6, and 24 weeks of age. B–D, bar graphs show quantification of the tissue cross-sectional area (CSA) (B), cortical thickness (Cort. Thickness) (C), and polar moment of inertia (pMOI) (D). E, representative microCT images from the femoral mid-diaphysis in control and ΔHif-1α;ΔHif-2α double mutants. Data are plotted mean ± S.E. with at least five mice being examined per genotype. *, p < 0.05
FIGURE 2.
Mechanical signals induce Hif-1α expression in osteocytes and osteoblasts. A and D, Hif-1α and Hif-2α levels were examined at the indicated times after MLO-Y4 osteocytes (A), and primary osteoblasts (D) were exposed to fluid flow for 1 h or left untreated (NT). B, exposing MLO-Y4 cells to hypoxia (2% O2) for 6 h increased both Hif-1α and Hif-2α protein levels. C and E, Hif-1α and osteopontin (Opn) mRNA levels were examined by quantitative PCR 8 h after MLO-Y4 cells (C) and primary osteoblasts (E) were exposed to fluid flow or left untreated.
FIGURE 3.
Prostanoid/mTOR signaling is required for induction of Hif-1α in response to fluid flow. A, Hif-1α, but not Hif-2α, protein levels were dose-dependently increased 8 h after treating MLO-Y4 cells with PGE2. B, NS398 (0.1 μ
m
) was used to inhibit PGE2 synthesis after fluid flow exposure. NT, untreated. C, blocking PGE2 synthesis inhibited the increase in Hif-1α protein levels after exposure to fluid flow, but not 10 μ
m
PGE2. D, both fluid flow and PGE2 induced the activation of Akt/mTOR signals as indicated by increased phosphorylation levels. p-Akt, phosphorylated Akt; p-mTOR, phosphorylated m-TOR; p-p70, phosphorylated p70. E and F, rapamycin (10 n
m
) abolished mTOR activity (E) and the effect of fluid flow and PGE2 on Hif-1α protein levels (F). G, disrupting mTOR expression abolished the induction of Hif-1α in primary osteoblasts. Data are plotted mean ± S.E. *, p < 0.05
FIGURE 4.
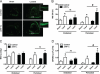
Loss of Hif-1α in osteoblasts enhances responsiveness to mechanical loading. The right tibia of 21-week-old female control and ΔHif-1α mice was subjected to a 3-week tibia loading regime, whereas the left tibia served as an unloaded, internal control (Sham). Both groups experienced equivalent peak periosteal strains. A, representative tissue sections for sham and loaded tibia in which calcein was injected on days 10 and 19 to assess bone formation. Magnified images are shown for the loaded samples. B–D, bar graphs show quantification of the mineralizing surface per bone surface (MS/BS) (B), mineral apposition rate (MAR) (C), and bone formation rate per bone surface (BFR/BS) (D) on both the periosteal and the endosteal surfaces. Data are plotted mean ± S.E. with eight mice being examined per genotype. *, p < 0.05 versus sham limb, # p < 0.05 versus wild-type control. ns, not significant.
FIGURE 5.
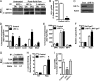
Hif-1α antagonizes load-induced β-catenin signaling. A, co-immunoprecipitation (IP) revealed that Hif-1α and β-catenin interact in both primary osteoblasts (Ob) and MLO-Y4 (Ocy) cells after exposure to fluid flow. B and C, primary osteoblasts isolated from Hif-1α floxed mice were infected with adenovirus expressing Cre recombinase to abolish Hif-1α expression (B) and prevent its induction after fluid flow exposure (C). Con, control. D and E, osteoblasts deficient for Hif-1α (ΔHif-1α) exhibited increased expression levels of β-catenin target genes (D), but not osteopontin (OPN), Cox-2, or IGF-1 (E), after exposure to fluid flow. F, BAT-gal reporter activity was increased in osteoblasts rendered deficient for Hif-1α and exposed to fluid flow. NT, untreated. G and H, an increased association of β-catenin with Tcf4 (G) and increased binding to the Axin2 promoter (H) were evident in ΔHif-1α osteoblasts after exposure to fluid flow in co-immunoprecipitation and ChIP assays, respectively. I, overexpressing Hif-1α by disrupting the expression of Vhl inhibited Axin2 expression after fluid flow exposure, whereas disrupting Hif-2α expression was without effect. Data are plotted mean ± S.E. *, p < 0.05
Similar articles
- Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice.
Bivi N, Pacheco-Costa R, Brun LR, Murphy TR, Farlow NR, Robling AG, Bellido T, Plotkin LI. Bivi N, et al. J Orthop Res. 2013 Jul;31(7):1075-81. doi: 10.1002/jor.22341. Epub 2013 Mar 11. J Orthop Res. 2013. PMID: 23483620 Free PMC article. - Rapid non-genomic signalling by 17β-oestradiol through c-Src involves mTOR-dependent expression of HIF-1α in breast cancer cells.
Sudhagar S, Sathya S, Lakshmi BS. Sudhagar S, et al. Br J Cancer. 2011 Sep 27;105(7):953-60. doi: 10.1038/bjc.2011.349. Epub 2011 Sep 6. Br J Cancer. 2011. PMID: 21897387 Free PMC article. - Osteocytes and WNT: the mechanical control of bone formation.
Galli C, Passeri G, Macaluso GM. Galli C, et al. J Dent Res. 2010 Apr;89(4):331-43. doi: 10.1177/0022034510363963. Epub 2010 Mar 3. J Dent Res. 2010. PMID: 20200416 Review. - Hypoxia Signaling in the Skeleton: Implications for Bone Health.
Yellowley CE, Genetos DC. Yellowley CE, et al. Curr Osteoporos Rep. 2019 Feb;17(1):26-35. doi: 10.1007/s11914-019-00500-6. Curr Osteoporos Rep. 2019. PMID: 30725321 Free PMC article. Review.
Cited by
- Overexpression of miR-223 Tips the Balance of Pro- and Anti-hypertrophic Signaling Cascades toward Physiologic Cardiac Hypertrophy.
Yang L, Li Y, Wang X, Mu X, Qin D, Huang W, Alshahrani S, Nieman M, Peng J, Essandoh K, Peng T, Wang Y, Lorenz J, Soleimani M, Zhao ZQ, Fan GC. Yang L, et al. J Biol Chem. 2016 Jul 22;291(30):15700-13. doi: 10.1074/jbc.M116.715805. Epub 2016 May 20. J Biol Chem. 2016. PMID: 27226563 Free PMC article. - Adaptive and Injury Response of Bone to Mechanical Loading.
McBride SH, Silva MJ. McBride SH, et al. Bonekey Osteovision. 2012 Oct 10;1:192. doi: 10.1038/bonekey.2012.192. Bonekey Osteovision. 2012. PMID: 23505338 Free PMC article. - Vascular patterning in human heterotopic ossification.
Cocks M, Mohan A, Meyers CA, Ding C, Levi B, McCarthy E, James AW. Cocks M, et al. Hum Pathol. 2017 May;63:165-170. doi: 10.1016/j.humpath.2017.03.005. Epub 2017 Mar 14. Hum Pathol. 2017. PMID: 28315426 Free PMC article. - CSF-1 in Osteocytes Inhibits Nox4-mediated Oxidative Stress and Promotes Normal Bone Homeostasis.
Werner SL, Sharma R, Woodruff K, Horn D, Harris SE, Gorin Y, Lee DY, Hua R, Gu S, Fajardo RJ, Habib SL, Jiang JX. Werner SL, et al. JBMR Plus. 2019 Jun 13;4(7):e10080. doi: 10.1002/jbm4.10080. eCollection 2020 Jul. JBMR Plus. 2019. PMID: 32666016 Free PMC article. - HIF-1α regulates bone formation after osteogenic mechanical loading.
Tomlinson RE, Silva MJ. Tomlinson RE, et al. Bone. 2015 Apr;73:98-104. doi: 10.1016/j.bone.2014.12.015. Epub 2014 Dec 23. Bone. 2015. PMID: 25541207 Free PMC article.
References
- Hillam R. A., Skerry T. M. (1995) J. Bone Miner. Res. 10, 683–689 - PubMed
- Pead M. J., Skerry T. M., Lanyon L. E. (1988) J. Bone Miner. Res 3, 647–656 - PubMed
- Morey E. R., Baylink D. J. (1978) Science 201, 1138–1141 - PubMed
- Bakker A. D., Soejima K., Klein-Nulend J., Burger E. H. (2001) J. Biomech. 34, 671–677 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous