An essential role for the circadian-regulated gene nocturnin in osteogenesis: the importance of local timekeeping in skeletal homeostasis - PubMed (original) (raw)
Comparative Study
An essential role for the circadian-regulated gene nocturnin in osteogenesis: the importance of local timekeeping in skeletal homeostasis
Anyonya R Guntur et al. Ann N Y Acad Sci. 2011 Nov.
Abstract
The role of circadian proteins in regulating whole-body metabolism and bone turnover has been studied in detail and has led to the discovery of an elemental system for timekeeping involving the core genes Clock, Bmal1, Per, and Cry. Nocturnin (Noc; Ccrn4l), a peripheral circadian-regulated gene has been shown to play a very important role in regulating adipogenesis by deadenylation of key mRNAs and intracytoplasmic transport of PPARγ. The role that it plays in osteogenesis has previously not been studied in detail. In this report we examined in vitro and in vivo osteogenesis in the presence and absence of Noc and show that loss of Noc enhances bone formation and can rescue rosiglitazone-induced bone loss in mice. The circadian rhythm of Noc is likely to be an essential element of marrow stromal cell fate.
© 2011 New York Academy of Sciences.
Conflict of interest statement
Conflicts of interest
The authors declare no conflicts of interests
Figures
Figure 1
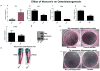
(A) Effect of Nocturnin on osteoblastogenesis. This figure shows effect of overexpression of (a) Noc on osteoblast gene marker expression as can be observed, there is a significant suppression of osteoblastogeneis, which is evident from the decrease in (b) ATF4 and (c) Osteocalci ; (d) Osterix we also saw a trend towards suppression in (e) CYP51 when Noc is overexpressed, in MC3T3E1 cells compared to GFP cells. (B) The increase in Noc is confirmed by the Flag tag Western blot along with a NOC protein levels in cells, Actin was used as the loading control. (C) (c’)The long term (20 days with change in media every two days) osteoblast differentiation assay on cells that have Noc knocked down show that there is a significant increase in nodule formation compared to control cells. (c’’) Osteoblast differentiation assay for 7 days showed decreased alkaline phosphatase staining in Noc overexpressed cells compared to control cells (n = 3, * indicates P < 0.05). (D) Alizarin red and Alcian blue staining showing the tibial and fibula bones from Noc control +/− and Noc null.
Figure 2
Effect of rosiglitazone treatment on bone in the absence of Nocturnin. Graphical representation of the data from mice that were on control diet and rosiglitazone diet. (a) Trabecular BV/TV, (b) trabecular connectivity density, (c) trabecular number, and (d) trabecular space. MicroCT evaluation of femurs from mice that were on rosiglitazone diet and compared to mice on control diet showed that there are increased trabecular parameters in the Noc null mice, suggesting that there is a protection effect of rosiglitazone treatment on bone in the absence of Noc. (n = 4 animals per group, _P_-values are indicated on the chart)
Figure 3
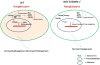
Model showing the effect rosiglitazone treatment has on bone in the presence and absence of Nocturnin. The model illustrates the salient points of the paper: in a wild-type (WT) pre-osteoblast in the presence of rosiglitazone, Nocturnin helps PPARγ translocate in to the nucleus where it can help facilitate adipogenesis potentially over osteogenesis; and in the cytoplasm, Nocturnin can potentially bind to the mRNA’s of ATF4, Osterix, and CYP51 and negatively regulate them using its deadenylase function. In the absence of Nocturnin (Nocturnin−/−) there is less of PPARγ in the nucleus, along with loss of deadenylase regulation of the osteoblast marker transcripts, which results in protection from bone loss on rosiglitazone treatment.
Similar articles
- A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation.
Kawai M, Green CB, Lecka-Czernik B, Douris N, Gilbert MR, Kojima S, Ackert-Bicknell C, Garg N, Horowitz MC, Adamo ML, Clemmons DR, Rosen CJ. Kawai M, et al. Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10508-13. doi: 10.1073/pnas.1000788107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498072 Free PMC article. - Nocturnin: a circadian target of Pparg-induced adipogenesis.
Kawai M, Green CB, Horowitz M, Ackert-Bicknell C, Lecka-Czernik B, Rosen CJ. Kawai M, et al. Ann N Y Acad Sci. 2010 Mar;1192:131-8. doi: 10.1111/j.1749-6632.2009.05221.x. Ann N Y Acad Sci. 2010. PMID: 20392228 Free PMC article. - PPARγ: a circadian transcription factor in adipogenesis and osteogenesis.
Kawai M, Rosen CJ. Kawai M, et al. Nat Rev Endocrinol. 2010 Nov;6(11):629-36. doi: 10.1038/nrendo.2010.155. Epub 2010 Sep 7. Nat Rev Endocrinol. 2010. PMID: 20820194 Free PMC article. Review. - Posttranscriptional regulation of mammalian circadian clock output.
Garbarino-Pico E, Green CB. Garbarino-Pico E, et al. Cold Spring Harb Symp Quant Biol. 2007;72:145-56. doi: 10.1101/sqb.2007.72.022. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419272 Review. - Nocturnin: at the crossroads of clocks and metabolism.
Stubblefield JJ, Terrien J, Green CB. Stubblefield JJ, et al. Trends Endocrinol Metab. 2012 Jul;23(7):326-33. doi: 10.1016/j.tem.2012.03.007. Epub 2012 May 17. Trends Endocrinol Metab. 2012. PMID: 22608110 Free PMC article. Review.
Cited by
- Differential processing and localization of human Nocturnin controls metabolism of mRNA and nicotinamide adenine dinucleotide cofactors.
Abshire ET, Hughes KL, Diao R, Pearce S, Gopalakrishna S, Trievel RC, Rorbach J, Freddolino PL, Goldstrohm AC. Abshire ET, et al. J Biol Chem. 2020 Oct 30;295(44):15112-15133. doi: 10.1074/jbc.RA120.012618. Epub 2020 Aug 23. J Biol Chem. 2020. PMID: 32839274 Free PMC article. - The structure of human Nocturnin reveals a conserved ribonuclease domain that represses target transcript translation and abundance in cells.
Abshire ET, Chasseur J, Bohn JA, Del Rizzo PA, Freddolino PL, Goldstrohm AC, Trievel RC. Abshire ET, et al. Nucleic Acids Res. 2018 Jul 6;46(12):6257-6270. doi: 10.1093/nar/gky412. Nucleic Acids Res. 2018. PMID: 29860338 Free PMC article. - Regulatory roles of vertebrate Nocturnin: insights and remaining mysteries.
Hughes KL, Abshire ET, Goldstrohm AC. Hughes KL, et al. RNA Biol. 2018;15(10):1255-1267. doi: 10.1080/15476286.2018.1526541. Epub 2018 Oct 9. RNA Biol. 2018. PMID: 30257600 Free PMC article. - Low intensity near-infrared light promotes bone regeneration via circadian clock protein cryptochrome 1.
Peng J, Zhao J, Tang Q, Wang J, Song W, Lu X, Huang X, Chen G, Zheng W, Zhang L, Han Y, Yan C, Wan Q, Chen L. Peng J, et al. Int J Oral Sci. 2022 Nov 14;14(1):53. doi: 10.1038/s41368-022-00207-y. Int J Oral Sci. 2022. PMID: 36376275 Free PMC article. - Pulmonary and hemostatic toxicity of multi-walled carbon nanotubes and zinc oxide nanoparticles after pulmonary exposure in Bmal1 knockout mice.
Luyts K, Smulders S, Napierska D, Van Kerckhoven S, Poels K, Scheers H, Hemmeryckx B, Nemery B, Hoylaerts MF, Hoet PH. Luyts K, et al. Part Fibre Toxicol. 2014 Nov 14;11:61. doi: 10.1186/s12989-014-0061-5. Part Fibre Toxicol. 2014. PMID: 25394423 Free PMC article.
References
- Phinney DG, Prockop DJ. Concise Review: Mesenchymal Stem/Multipotent Stromal Cells: The State of Transdifferentiation and Modes of Tissue Repair—Current Views. STEM CELLS. 2007;25:2896–2902. - PubMed
- Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Current Opinion in Pharmacology. 2004;4:290–294. - PubMed
- Fu L, Patel MS, et al. The Molecular Clock Mediates Leptin-Regulated Bone Formation. Cell. 2005;122(5):803–815. - PubMed
- Froy O. Metabolism and Circadian Rhythms—Implications for Obesity. Endocrine Reviews. 2010;31:1–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR054604-04/AR/NIAMS NIH HHS/United States
- R01 AR054604/AR/NIAMS NIH HHS/United States
- R01 AR045433/AR/NIAMS NIH HHS/United States
- R01 AR045433-15/AR/NIAMS NIH HHS/United States
- AR54604/AR/NIAMS NIH HHS/United States
- AR045433/AR/NIAMS NIH HHS/United States
- P20RR18789/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases