Aspirin in the chemoprevention of colorectal neoplasia: an overview - PubMed (original) (raw)
Review
Aspirin in the chemoprevention of colorectal neoplasia: an overview
Andrew T Chan et al. Cancer Prev Res (Phila). 2012 Feb.
Abstract
Considerable evidence supports the effectiveness of aspirin for chemoprevention of colorectal cancer (CRC) in addition to its well-established benefits in the prevention of vascular disease. Epidemiologic studies have consistently observed an inverse association between aspirin use and risk of CRC. A recent pooled analysis of a long-term posttrial follow-up of nearly 14,000 patients from four randomized, cardiovascular disease prevention trials showed that daily aspirin treatment for about five years was associated with a 34% reduction in 20-year CRC mortality. A separate metaanalysis of nearly 3,000 patients with a history of colorectal adenoma or cancer in four randomized adenoma prevention trials showed that aspirin reduced the occurrence of advanced adenomas by 28% and any adenoma by 17%. Aspirin has also been shown to be beneficial in a clinical trial of patients with Lynch syndrome, a hereditary CRC syndrome; in those treated with aspirin for at least two years, there was a 50% or more reduction in the risk of CRC commencing five years after randomization and after aspirin had been discontinued. A few observational studies have shown an increase in survival among patients with CRC who use aspirin. Taken together, these findings strengthen the case for consideration of long-term aspirin use in CRC prevention. Despite these compelling data, there is a lack of consensus about the balance of risks and benefits associated with long-term aspirin use, particularly in low-risk populations. The optimal dose to use for cancer prevention and the precise mechanism underlying aspirin's anticancer effect require further investigation.
©2011 AACR.
Figures
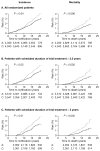
Figure 1
Pooled analysis of the effect of aspirin (thick line) versus control (thin line) on subsequent incidence and mortality due to colorectal cancer in all randomized patients (A) in three trials of low-dose aspirin versus placebo, in those with scheduled duration of trial treatment ≥ 2.5 years (B), and in those with scheduled duration of trial treatment ≥ 5 years (C). A, aspirin; C, control. Reprinted from The Lancet, 376, Rothwell P, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials, 1741-50, Copyright 2010, with permission from Elsevier.
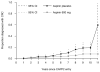
Figure 2
CAPP2: Time to first colorectal cancer in those randomized to aspirin compared with those randomized to the aspirin placebo. In each case, Kaplan-Meier analysis was restricted to participants who had taken ≥ 2 years’ intervention and the analysis was adjusted for gender (hazard ratio 0.41 (95% confidence interval, 0.19–0·86), P = 0·02). Each point on the plot shows the estimated cumulative incidence by years of follow-up, together with the corresponding 95% confidence interval.
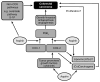
Figure 3
Possible sites of action of aspirin in the prevention of colorectal cancer. COX-dependent and independent targets of aspirin are likely to be present in both epithelial and stromal cell compartments in colorectal adenomas and cancers. ATLs, aspirin-triggered lipoxins; COX, cyclooxygenase; PGE2, prostaglandin E2; S-1P, sphingosine-1-phosphate.
References
- GLOBOCAN 2008. Feb 2, 2011. p. 2011.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. - PubMed
- Screening for colorectal cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. - PubMed
- National Comprehensive Cancer Network. Colorectal cancer screening. V1, 2010. Vol. 2011. Feb 2, 2011.
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 09/100/25/DH_/Department of Health/United Kingdom
- G116/146(60953)/MRC_/Medical Research Council/United Kingdom
- EME/09/100/25/DH_/Department of Health/United Kingdom
- R01 CA137178/CA/NCI NIH HHS/United States
- G116/146/MRC_/Medical Research Council/United Kingdom
- R01 CA059005/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical