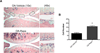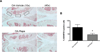Autophagy activation by rapamycin reduces severity of experimental osteoarthritis - PubMed (original) (raw)
Autophagy activation by rapamycin reduces severity of experimental osteoarthritis
Beatriz Caramés et al. Ann Rheum Dis. 2012 Apr.
Abstract
Objectives: Osteoarthritis is associated with cell death and extracellular matrix degradation in articular cartilage. Autophagy is an essential cellular homeostasis mechanism that was found to be deficient in ageing and osteoarthritic cartilage. This study determined whether pharmacological inhibition of the mammalian target of rapamycin (mTOR), a key inhibitor of autophagy, has disease-modifying activity in experimental osteoarthritis.
Methods: Experimental osteoarthritis was induced by transection of the medial meniscotibial ligament and the medial collateral ligament in 2-month-old C57Bl/6 mice (n=36). Rapamycin (1 mg/kg weight/day) (n=18 mice) or dimethyl sulphoxide vehicle control (n=18 mice) was administered intraperitoneally for 10 weeks. Histopathological changes in articular cartilage and synovium were examined by using semiquantitative scoring systems. Rapamycin effects on mTOR signalling, autophagy, cartilage homeostasis and inflammation were analysed by immunohistochemistry and immunofluorescence staining.
Results: Rapamycin affected the mTOR signalling pathway in mouse knee joints as indicated by the inhibition of ribosomal protein S6 phosphorylation, a target of mTOR and activation of LC3, a main marker of autophagy. The severity of cartilage degradation was significantly (p<0.01) reduced in the rapamycin-treated group compared with the control group and this was associated with a significant (p<0.05) decrease in synovitis. Rapamycin treatment also maintained cartilage cellularity and decreased ADAMTS-5 and interleukin-1β expression in articular cartilage.
Conclusions: These results suggest that rapamycin, at least in part by autophagy activation, reduces the severity of experimental osteoarthritis. Pharmacological activation of autophagy may be an effective therapeutic approach for osteoarthritis.
Figures
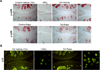
Figure 1. Systemic administration of rapamycin modulates mTOR signaling and autophagy in mouse knee joints
A, Knee joints from C57Bl/6J mice were collected 10 weeks after knee destabilization or and treatment with rapamycin (rapa) or vehicle (n=3/each). Contralateral knees were used as a control. Sections were analyzed by immunohistochemistry for phosphorylation of ribosomal protein rpS6. Magnification: ×10 and ×40. B, Knee joints from 2 months old C57Bl/6J mice after OA surgery treated with vehicle (n=4) or rapa (n=4) were analyzed by immunofluorescence for LC3. Magnification: 10× and 100×.
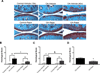
Figure 2. Rapamycin reduces severity of experimental OA
Two months old C57Bl/6J mice were subjected to OA surgery in right the knee. The left knee was not subjected to surgery and used as control. Two separate experiments were performed. Each experiment included 18 mice, with 9 receiving rapamycin (rapa) and 9 receiving vehicle. A, Knee joints were analyzed by staining with Safranin O. Magnification:10x, 40x. B–C, Histological scores for the two separate experiments. Values are mean ± SD. * = P < 0.01 versus control-vehicle and control-rapa; ** = P < 0.01 versus OA-vehicle. D, Histological scores after combining both experiments (n= 36) showed a significant decrease in OA scores after rapa treatment. Values are the mean ± SD. * = P < 0.01 versus OA-vehicle.
Figure 3. Rapamycin prevents reduction in cartilage cellularity during experimental OA
A, Knee joint sections from C57Bl/6J mice with or without OA surgery under treatment with rapamycin (rapa) or vehicle (n=6 each) were stained with Hematoxylin Eosin (H&E). Magnification: 10× and 40×. B, Quantitative analysis of cell number showed a significant increase of cellularity after rapa treatment compared to vehicle treatment. Values are the mean ± SD. * = P < 0.001 versus OA-vehicle.
Figure 4. Rapamycin reduces ADAMTS-5 expression. A
Knee joints from C57Bl/6J mice were collected 10 weeks after OA surgery and treatment with rapamycin (rapa) or vehicle (n=5 each). Sections were analyzed by immunohistochemistry for ADAMTS-5. Magnification: 10× and 40×. B, Quantitative analysis of ADAMTS-5 positive cells. Total cell number in three fields and ADAMTS-5 positive cells were counted and the percentage of ADAMTS-5 positive cells was calculated. Results show a significant decrease in ADAMTS-5 after rapa treatment compared to vehicle. Values are mean ± SD. * = P < 0.05 versus OA-vehicle.
Figure 5. Effect of Rapamycin on inflammation in experimental OA
A, Synovium from mice after experimental OA and vehicle (N= 16) or rapamycin (rapa, N= 11) treatment was analyzed by staining with Safranin O. Magnification: 40×. B, The histological score for inflammation was significantly decreased after rapa treatment. Values are the mean ± SD. * = P < 0.05 versus OA-vehicle. C, Synovium sections from vehicle or rapa treated mice (N=5 each) were analyzed by immunohistochemistry for IL-1β. Magnification: 40×.
Similar articles
- Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis.
Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP, Martel-Pelletier J, Kapoor M. Zhang Y, et al. Ann Rheum Dis. 2015 Jul;74(7):1432-40. doi: 10.1136/annrheumdis-2013-204599. Epub 2014 Mar 20. Ann Rheum Dis. 2015. PMID: 24651621 - Rapamycin Inhibits Nf-ΚB Activation by Autophagy to Reduce Catabolism in Human Chondrocytes.
Liu Y, Li X, Jin A. Liu Y, et al. J Invest Surg. 2020 Oct;33(9):861-873. doi: 10.1080/08941939.2019.1574321. Epub 2019 Apr 4. J Invest Surg. 2020. PMID: 30945580 - Enhancement of the synthesis of n-3 PUFAs in fat-1 transgenic mice inhibits mTORC1 signalling and delays surgically induced osteoarthritis in comparison with wild-type mice.
Huang MJ, Wang L, Jin DD, Zhang ZM, Chen TY, Jia CH, Wang Y, Zhen XC, Huang B, Yan B, Chen YH, Li SF, Yang JC, Dai YF, Bai XC. Huang MJ, et al. Ann Rheum Dis. 2014 Sep;73(9):1719-27. doi: 10.1136/annrheumdis-2013-203231. Epub 2013 Jul 12. Ann Rheum Dis. 2014. PMID: 23852692 - Autophagy in osteoarthritis.
Li YS, Zhang FJ, Zeng C, Luo W, Xiao WF, Gao SG, Lei GH. Li YS, et al. Joint Bone Spine. 2016 Mar;83(2):143-8. doi: 10.1016/j.jbspin.2015.06.009. Epub 2015 Oct 6. Joint Bone Spine. 2016. PMID: 26453105 Review. - mTOR: a potential therapeutic target in osteoarthritis?
Pal B, Endisha H, Zhang Y, Kapoor M. Pal B, et al. Drugs R D. 2015 Mar;15(1):27-36. doi: 10.1007/s40268-015-0082-z. Drugs R D. 2015. PMID: 25688060 Free PMC article. Review.
Cited by
- Differences in Mammalian target of rapamycin gene expression in the peripheral blood and articular cartilages of osteoarthritic patients and disease activity.
Tchetina EV, Poole AR, Zaitseva EM, Sharapova EP, Kashevarova NG, Taskina EA, Alekseeva LI, Semyonova LA, Glukhova SI, Kuzin AN, Makarov MA, Makarov SA. Tchetina EV, et al. Arthritis. 2013;2013:461486. doi: 10.1155/2013/461486. Epub 2013 Jun 25. Arthritis. 2013. PMID: 23864948 Free PMC article. - PPARγ/mTOR signalling: striking the right balance in cartilage homeostasis.
Dell'Accio F, Sherwood J. Dell'Accio F, et al. Ann Rheum Dis. 2015 Mar;74(3):477-9. doi: 10.1136/annrheumdis-2014-206884. Epub 2015 Jan 14. Ann Rheum Dis. 2015. PMID: 25589512 Free PMC article. No abstract available. - Autophagy attenuates the catabolic effect during inflammatory conditions in nucleus pulposus cells, as sustained by NF-κB and JNK inhibition.
Xu K, Chen W, Wang X, Peng Y, Liang A, Huang D, Li C, Ye W. Xu K, et al. Int J Mol Med. 2015 Sep;36(3):661-8. doi: 10.3892/ijmm.2015.2280. Epub 2015 Jul 10. Int J Mol Med. 2015. PMID: 26165348 Free PMC article. - The effects of metformin in the treatment of osteoarthritis: Current perspectives.
Song Y, Wu Z, Zhao P. Song Y, et al. Front Pharmacol. 2022 Aug 23;13:952560. doi: 10.3389/fphar.2022.952560. eCollection 2022. Front Pharmacol. 2022. PMID: 36081941 Free PMC article. Review. - Functional annotation of rheumatoid arthritis and osteoarthritis associated genes by integrative genome-wide gene expression profiling analysis.
Li ZC, Xiao J, Peng JL, Chen JW, Ma T, Cheng GQ, Dong YQ, Wang WL, Liu ZD. Li ZC, et al. PLoS One. 2014 Feb 14;9(2):e85784. doi: 10.1371/journal.pone.0085784. eCollection 2014. PLoS One. 2014. PMID: 24551036 Free PMC article.
References
- Takayama K, Ishida K, Matsushita T, et al. SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum. 2009;60:2731–2740. - PubMed
- Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. - PubMed
- Hellio Le Graverand-Gastineau MP. OA clinical trials: current targets and trials for OA Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC2 AR058954-02/AR/NIAMS NIH HHS/United States
- R01 AR056026/AR/NIAMS NIH HHS/United States
- S10 RR027557/RR/NCRR NIH HHS/United States
- RR027577/RR/NCRR NIH HHS/United States
- RC2 AR058954/AR/NIAMS NIH HHS/United States
- S10 RR027557-01/RR/NCRR NIH HHS/United States
- AR058954/AR/NIAMS NIH HHS/United States
- P01 AG007996-18/AG/NIA NIH HHS/United States
- AG007996/AG/NIA NIH HHS/United States
- P01 AG007996/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous