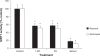Effects of Kynurenine Pathway Inhibition on NAD Metabolism and Cell Viability in Human Primary Astrocytes and Neurons - PubMed (original) (raw)
Effects of Kynurenine Pathway Inhibition on NAD Metabolism and Cell Viability in Human Primary Astrocytes and Neurons
Nady Braidy et al. Int J Tryptophan Res. 2011.
Abstract
The kynurenine pathway (KP) is the principle route of L-Tryptophan (TRP) metabolism, producing several neurotoxic and neuroprotective metabolic precursors before complete oxidation to the essential pyridine nucleotide nicotinamide adenine dinucleotide (NAD(+)). KP inhibition may prove therapeutic in central nervous system (CNS) inflammation by reducing the production of excitotoxins such as quinolinic acid (QUIN). However, KP metabolism may also be cytoprotective through the de novo synthesis of intracellular NAD(+). We tested the hypothesis that the KP is directly involved in the maintenance of intracellular NAD(+) levels and SIRT1 function in primary astrocytes and neurons through regulation of NAD(+) synthesis. Competitive inhibition of indoleamine 2,3 dioxygenase (IDO), and quinolinic acid phosphoribosyltransferase (QPRT) activities with 1-methyl-L-Tryptophan (1-MT), and phthalic acid (PA) respectively, resulted in a dose-dependent decrease in intracellular NAD(+) levels and sirtuin deacetylase-1 (SIRT1) activity, and correlated directly with reduced cell viability. These results support the hypothesis that the primary role of KP activation during neuroinflammation is to maintain NAD(+) levels through de novo synthesis from TRP. Inhibition of KP metabolism under these conditions can compromise cell viability, NAD-dependent SIRT1 activity and CNS function, unless alternative precursors for NAD(+) synthesis are made available.
Keywords: IDO; NAD+; astrocytes neurons 1-MT; sirtuins.
Figures
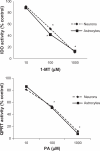
Figure 1
A) Effect of 1-MT on IDO activity in human astrocytes and neurons. A dose-dependent inhibition of IDO activity was observed following treatment with 1-MT in human astrocytes and neurons. For astrocytes, no 1-MT (control) = 35.86 nmol kynurenine/hr/mg protein; 10 μM 1-MT = 31.15 ± 5.61 nmol kynurenine/hr/mg protein; 100 μM 1-MT = 14.70 ± 4.85 nmol kynurenine/hr/mg protein; 1000 μM 1-MT = 4.55 ± 1.93 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). For neurons, no 1-MT (control) = 27.22 ± 7.28 nmol kynurenine/hr/mg protein; 10 μM 1-MT = 24.77 ± 6.74 nmol kynurenine/hr/mg protein; 100 μM 1-MT = 14.15 ± 2.94 nmol kynurenine/hr/mg protein; 1000 μM 1-MT = 2.99 ± 1.42 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). B) PA on QPRT activity in human astrocytes and neurons. A dose-dependent inhibition of QPRT activity was observed following treatment with PA in human astrocytes and neurons. For astrocytes, no PA (control) = 41.33 ± 8.32 nmol kynurenine/hr/mg protein; 10 μM PA = 35.54 ± 3.22 nmol kynurenine/hr/mg protein; 100 μM PA = 21.08 ± 7.39 nmol kynurenine/hr/mg protein; 1000 μM PA = 3.31 ± 1.32 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group). For neurons, no PA (control) = 21.55 ± 3.62 nmol kynurenine/hr/mg protein; 10 μM PA = 17.46 ± 3.49 nmol kynurenine/hr/mg protein; 100 μM PA = 11.42 ± 3.11 nmol kynurenine/hr/mg protein; 1000 μM PA = 2.59 ± 0.81 nmol kynurenine/hr/mg protein; Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
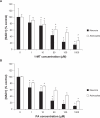
Figure 2
Effect of (A) 1-MT and (B) PA on intracellular NAD+ levels in human astrocytes and neurons. NAD+ levels significantly declined in a dose-dependent manner with increasing concentrations of (A) 1-MT and (B) PA respectively following 24 hours incubation with the selected inhibitor. Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
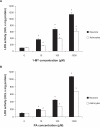
Figure 3
Effect of (A) 1-MT and (B) PA on extracellular LDH activity in human astrocyte and neuron cultures. Extracellular LDH activities were significantly elevated in a dose-dependent manner with increasing concentrations of (A) 1-MT and (B) PA respectively, following 24 hours incubation with the selected inhibitor. Significance *P < 0.05 compared to previous dose (n = 4 for each treatment group).
Figure 4
Effect of 1-MT and PA on SIRT1 activity in human astrocytes and neurons. Treatment with 1-MT or PA at 100 μM significantly reduced SIRT1 activity in human astrocytes and neurons. Significance *P < 0.05 compared to control (n = 4 for each treatment group).
Similar articles
- Inhibition of indoleamine 2,3-dioxygenase activity in IFN-gamma stimulated astroglioma cells decreases intracellular NAD levels.
Grant R, Kapoor V. Grant R, et al. Biochem Pharmacol. 2003 Sep 15;66(6):1033-6. doi: 10.1016/s0006-2952(03)00464-7. Biochem Pharmacol. 2003. PMID: 12963490 - Effects of Kynurenine Pathway Metabolites on Intracellular NAD Synthesis and Cell Death in Human Primary Astrocytes and Neurons.
Braidy N, Grant R, Brew BJ, Adams S, Jayasena T, Guillemin GJ. Braidy N, et al. Int J Tryptophan Res. 2009;2:61-9. doi: 10.4137/ijtr.s2318. Epub 2009 Apr 3. Int J Tryptophan Res. 2009. PMID: 22084582 Free PMC article. - Quinolinate as a Marker for Kynurenine Metabolite Formation and the Unresolved Question of NAD+ Synthesis During Inflammation and Infection.
Moffett JR, Arun P, Puthillathu N, Vengilote R, Ives JA, Badawy AA, Namboodiri AM. Moffett JR, et al. Front Immunol. 2020 Feb 21;11:31. doi: 10.3389/fimmu.2020.00031. eCollection 2020. Front Immunol. 2020. PMID: 32153556 Free PMC article. - Kynurenine pathway in Parkinson's disease-An update.
Venkatesan D, Iyer M, Narayanasamy A, Siva K, Vellingiri B. Venkatesan D, et al. eNeurologicalSci. 2020 Sep 10;21:100270. doi: 10.1016/j.ensci.2020.100270. eCollection 2020 Dec. eNeurologicalSci. 2020. PMID: 33134567 Free PMC article. Review. - The kynurenine pathway in brain tumor pathogenesis.
Adams S, Braidy N, Bessede A, Brew BJ, Grant R, Teo C, Guillemin GJ. Adams S, et al. Cancer Res. 2012 Nov 15;72(22):5649-57. doi: 10.1158/0008-5472.CAN-12-0549. Epub 2012 Nov 9. Cancer Res. 2012. PMID: 23144293 Review.
Cited by
- Distinct Capabilities in NAD Metabolism Mediate Resistance to NAMPT Inhibition in Glioblastoma.
Perryman R, Chau TW, De-Felice J, O'Neill K, Syed N. Perryman R, et al. Cancers (Basel). 2024 May 29;16(11):2054. doi: 10.3390/cancers16112054. Cancers (Basel). 2024. PMID: 38893173 Free PMC article. - Major Developments in the Design of Inhibitors along the Kynurenine Pathway.
Jacobs KR, Castellano-Gonzalez G, Guillemin GJ, Lovejoy DB. Jacobs KR, et al. Curr Med Chem. 2017;24(23):2471-2495. doi: 10.2174/0929867324666170502123114. Curr Med Chem. 2017. PMID: 28464785 Free PMC article. Review. - Effects of Ozone on Sickness and Depressive-like Behavioral and Biochemical Phenotypes and Their Regulation by Serum Amyloid A in Mice.
Baumann KK, Liang WS, Quaranta DV, Wilson ML, Asrat HS, Thysell JA, Sarchi AV, Banks WA, Erickson MA. Baumann KK, et al. Int J Mol Sci. 2023 Jan 13;24(2):1612. doi: 10.3390/ijms24021612. Int J Mol Sci. 2023. PMID: 36675130 Free PMC article. - Kynurenine 3-Monooxygenase Activity in Human Primary Neurons and Effect on Cellular Bioenergetics Identifies New Neurotoxic Mechanisms.
Castellano-Gonzalez G, Jacobs KR, Don E, Cole NJ, Adams S, Lim CK, Lovejoy DB, Guillemin GJ. Castellano-Gonzalez G, et al. Neurotox Res. 2019 Apr;35(3):530-541. doi: 10.1007/s12640-019-9997-4. Epub 2019 Jan 21. Neurotox Res. 2019. PMID: 30666558 - Association of urinary concentrations of phthalate metabolites with quinolinic acid among women: A potential link to neurological disorders.
Nassan FL, Gunn JA, Hill MM, Williams PL, Hauser R. Nassan FL, et al. Environ Int. 2020 May;138:105643. doi: 10.1016/j.envint.2020.105643. Epub 2020 Mar 14. Environ Int. 2020. PMID: 32179323 Free PMC article.
References
- Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64:185–218. - PubMed
- Stone TW. Purines and neuroprotection. Adv Exp Med Biol. 2002;513:249–80. - PubMed
- Heyes MP. The kynurenine pathway and neurologic disease. Therapeutic strategies. Adv Exp Med Biol. 1996;398:125–9. - PubMed
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer’s disease. Int J Biochem Cell Biol. 2005;37:289–305. - PubMed
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials