The potential for crizotinib in non-small cell lung cancer: a perspective review - PubMed (original) (raw)
The potential for crizotinib in non-small cell lung cancer: a perspective review
Yung-Jue Bang. Ther Adv Med Oncol. 2011 Nov.
Abstract
Tyrosine kinases have a crucial role as key regulators of signaling pathways that influence cell differentiation and growth. Dysregulation of tyrosine kinase-mediated signaling is understood to be an important oncogenic driver. Genetic rearrangements involving the tyrosine kinase anaplastic lymphoma kinase (ALK) gene occur in non-small cell lung cancer (NSCLC), anaplastic large cell lymphomoas, inflammatory myofibroblastic tumors, and other cancers. Cells with abnormal ALK signaling are sensitive to ALK inhibitors such as crizotinib. This review will highlight the discovery of the fusion between echinoderm microtubule-associated protein-like 4 (EML4) and ALK as an oncogenic driver, recognition of other ALK gene rearrangements in NSCLC, and the confirmation that crizotinib is an effective treatment for patients with ALK-positive NSCLC. Work is underway to further define the role for crizotinib in the treatment of ALK-positive lung cancer and other cancers and to investigate the molecular mechanisms for resistance to ALK inhibition with crizotinib.
Keywords: anaplastic lymphoma kinase; carcinoma; crizotinib; non-small cell lung cancer; tyrosine kinase inhibitor.
Figures
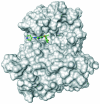
Figure 1.
Crizotinib in the anaplastic lymphoma kinase ATP binding pocket [Camidge et al. 2010a].
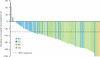
Figure 2.
Waterfall plot of best percentage change from baseline in target lesions by patient in patients with anaplastic lymphoma kinase (ALK)_-_positive non-small cell lung cancer who received crizotinib (excluding those with early death and indeterminate or unavailable response from the 105 evaluable patients; August 2010) [Camidge et al. 2010a]. CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 3.
Time to response with crizotinib for responding patients (n = 59 of 105 evaluable patients; August 2010) [Solomon et al. 2010].
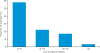
Figure 4.
Progression-free survival (PFS) following treatment with crizotinib (August 2010; N = 113) [Camidge et al. 2010a]. CI, confidence interval.
Similar articles
- ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC.
Casaluce F, Sgambato A, Maione P, Rossi A, Ferrara C, Napolitano A, Palazzolo G, Ciardiello F, Gridelli C. Casaluce F, et al. Target Oncol. 2013 Mar;8(1):55-67. doi: 10.1007/s11523-012-0250-9. Epub 2013 Jan 17. Target Oncol. 2013. PMID: 23325296 Review. - ALK inhibitors in the treatment of advanced NSCLC.
Gridelli C, Peters S, Sgambato A, Casaluce F, Adjei AA, Ciardiello F. Gridelli C, et al. Cancer Treat Rev. 2014 Mar;40(2):300-6. doi: 10.1016/j.ctrv.2013.07.002. Epub 2013 Aug 7. Cancer Treat Rev. 2014. PMID: 23931927 Review. - Targeted therapies in non-small cell lung cancer: a focus on ALK/ROS1 tyrosine kinase inhibitors.
Sgambato A, Casaluce F, Maione P, Gridelli C. Sgambato A, et al. Expert Rev Anticancer Ther. 2018 Jan;18(1):71-80. doi: 10.1080/14737140.2018.1412260. Epub 2017 Dec 6. Expert Rev Anticancer Ther. 2018. PMID: 29187012 Review. - Anaplastic lymphoma kinase (ALK): structure, oncogenic activation, and pharmacological inhibition.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2013 Feb;68(1):68-94. doi: 10.1016/j.phrs.2012.11.007. Epub 2012 Nov 28. Pharmacol Res. 2013. PMID: 23201355 Review. - New insights into anaplastic lymphoma kinase-positive nonsmall cell lung cancer.
Dubey AP, Pathi N, Viswanath S, Rathore A, Pathak A, Sud R. Dubey AP, et al. Indian J Cancer. 2017 Jan-Mar;54(1):203-208. doi: 10.4103/ijc.IJC_72_17. Indian J Cancer. 2017. PMID: 29199691
Cited by
- Novel covalent modification of human anaplastic lymphoma kinase (ALK) and potentiation of crizotinib-mediated inhibition of ALK activity by BNP7787.
Parker AR, Petluru PN, Nienaber VL, Zhao M, Ayala PY, Badger J, Chie-Leon B, Sridhar V, Logan C, Kochat H, Hausheer FH. Parker AR, et al. Onco Targets Ther. 2015 Feb 4;8:375-83. doi: 10.2147/OTT.S73690. eCollection 2015. Onco Targets Ther. 2015. PMID: 25678804 Free PMC article. - Fluorescence in situ hybridization, immunohistochemistry, and next-generation sequencing for detection of EML4-ALK rearrangement in lung cancer.
Pekar-Zlotin M, Hirsch FR, Soussan-Gutman L, Ilouze M, Dvir A, Boyle T, Wynes M, Miller VA, Lipson D, Palmer GA, Ali SM, Dekel S, Brenner R, Bunn PA Jr, Peled N. Pekar-Zlotin M, et al. Oncologist. 2015 Mar;20(3):316-22. doi: 10.1634/theoncologist.2014-0389. Epub 2015 Feb 26. Oncologist. 2015. PMID: 25721120 Free PMC article. - TKI sensitivity patterns of novel kinase-domain mutations suggest therapeutic opportunities for patients with resistant ALK+ tumors.
Amin AD, Li L, Rajan SS, Gokhale V, Groysman MJ, Pongtornpipat P, Tapia EO, Wang M, Schatz JH. Amin AD, et al. Oncotarget. 2016 Apr 26;7(17):23715-29. doi: 10.18632/oncotarget.8173. Oncotarget. 2016. PMID: 27009859 Free PMC article. - ALK inhibitors: a new targeted therapy in the treatment of advanced NSCLC.
Casaluce F, Sgambato A, Maione P, Rossi A, Ferrara C, Napolitano A, Palazzolo G, Ciardiello F, Gridelli C. Casaluce F, et al. Target Oncol. 2013 Mar;8(1):55-67. doi: 10.1007/s11523-012-0250-9. Epub 2013 Jan 17. Target Oncol. 2013. PMID: 23325296 Review. - Immunologic and clinical effects of targeting PD-1 in lung cancer.
Harvey RD. Harvey RD. Clin Pharmacol Ther. 2014 Aug;96(2):214-23. doi: 10.1038/clpt.2014.74. Epub 2014 Apr 1. Clin Pharmacol Ther. 2014. PMID: 24690569 Free PMC article. Review.
References
- American Cancer Society (2011) Global Cancer: Facts and Figures, 2nd edn. American Cancer Society website: www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/doc... [last accessed 27 July 2011]
- Bang Y., Kway E.L., Shaw A.T., Camidge D.R., Iafrate A.J., Maki R.G., et al. (2010) Clinical activity of the oral ALK inhibitor PF-02341066 in ALK-positive patients with non-small cell lung cancer (NSCLC). Presented at the 46th American Society of Clinical Oncology Annual Meeting. J Clin Oncol 28(Suppl 18s) (Abstract 3)
- Boland J.M., Erdogan S., Vasmatzis G., Yang P., Tillmans L.S., Johnson M.R., et al. (2009) Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non-small cell lung carcinomas. Hum Pathol 40: 1152–1158 - PubMed
- Bustin S.A. (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25: 169–193 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous