3-Substituted pyrazole analogs of the cannabinoid type 1 (CB₁) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB₁, non-CB₂ mechanism - PubMed (original) (raw)
3-Substituted pyrazole analogs of the cannabinoid type 1 (CB₁) receptor antagonist rimonabant: cannabinoid agonist-like effects in mice via non-CB₁, non-CB₂ mechanism
Jenny L Wiley et al. J Pharmacol Exp Ther. 2012 Feb.
Abstract
The prototypic cannabinoid type 1 (CB₁) receptor antagonist/inverse agonist, rimonabant, is comprised of a pyrazole core surrounded by a carboxyamide with terminal piperidine group (3-substituent), a 2,4-dichlorophenyl group (1-substituent), a 4-chlorophenyl group (5-substituent), and a methyl group (4-substituent). Previous structure-activity relationship (SAR) analysis has suggested that the 3-position may be involved in receptor recognition and agonist activity. The goal of the present study was to develop CB₁-selective compounds and explore further the SAR of 3-substitution on the rimonabant template. 3-Substituted analogs with benzyl and alkyl amino, dihydrooxazole, and oxazole moieties were synthesized and evaluated in vitro and in vivo. Several notable patterns emerged. First, most of the analogs exhibited CB₁ selectivity, with many lacking affinity for the CB₂ receptor. Affinity tended to be better when [³H]5-(4-chlorophenyl)-1-(2,4-dichloro-phenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide (SR141716), rather than [³H](-)-cis-3-[2-hydroxy-4(1,1-dimethyl-heptyl)phenyl]-trans-4-(3-hydroxy-propyl)cyclohexanol (CP55,940), was used as the binding radioligand. Second, many of the analogs produced an agonist-like profile of effects in mice (i.e., suppression of activity, antinociception, hypothermia, and immobility); however, their potencies were not well correlated with their CB₁ binding affinities. Further assessment of selected analogs showed that none were effective antagonists of the effects of Δ⁹-tetrahydrocannabinol in mice, their agonist-like effects were not blocked by rimonabant, they were active in vivo in CB₁⁻/⁻ mice, and they failed to stimulate guanosine-5'-O-(3-[³⁵S]thio)-triphosphate binding. Several analogs were inverse agonists in the latter assay. Together, these results suggest that this series of 3-substituted pyrazole analogs represent a novel class of CB₁-selective cannabinoids that produce agonist-like effects in mice through a non-CB₁, non-CB₂ mechanism.
Figures
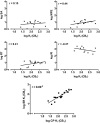
Fig. 1.
Top and middle, scatterplots and regression lines of CB1 affinities (log _K_i), measured by [3H]SR141716 displacement, plotted against log ED50 for each of the four in vivo tests (SA, spontaneous activity; RT, change in rectal temperature; RI, ring immobility). Bottom, scatterplot and regression line of CB1 affinities (log _K_i) as measured with two radioligands, the cannabinoid receptor agonist [3H]CP55,940 and the cannabinoid receptor antagonist [3H]SR141716. Pearson product-moment correlations are shown for the two measures graphed in each panel. * indicates significant correlation (p < 0.05).
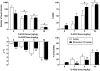
Fig. 2.
Effects of O-4332 in combination with vehicle (open bars) and 10 mg/kg rimonabant (filled bars) on locomotor activity expressed as number of photocell beam breaks (top left), antinociception expressed as percentage of maximum possible effect (top right), change in rectal temperature (bottom left), and percentage ring immobility (bottom right). Each bar represents the mean (± S.E.M.) of six mice. # indicates significant (p < 0.05) main effect of O-4332 dose compared with vehicle. * indicates significant interaction, with post hoc difference in effect of O-4332 dose compared with vehicle/vehicle condition. ** indicates significant interaction, with post hoc difference in effect of O-4332 and rimonabant combination compared with the same dose of O-4332 with vehicle.
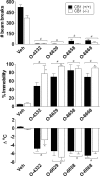
Fig. 3.
Effects of 10 mg/kg doses of selected pyrazole analogs (O-6211, O-6629, O-6658, and O-6668) in combination with vehicle (Veh) (open bars) and 10 mg/kg rimonabant (filled bars) on locomotor activity expressed as number of photocell beam breaks (top left), antinociception expressed as percentage of maximum possible effect (top right), change in rectal temperature (bottom left), and percentage of ring immobility (bottom right). Each bar represents the mean (± S.E.M.) of six mice, with the exception that n = 24 for vehicle/vehicle and rimonabant/vehicle groups and n = 4–5 for ring immobility measure for O-6211 and O-6668. # indicates significant (p < 0.05) main effect of the indicated compound compared with vehicle. * indicates significant interaction, with post hoc difference in effect of compound and rimonabant condition compared with vehicle and compound condition.
Fig. 4.
Effects of 30 mg/kg O-4332 and 10 mg/kg O-6629, O-6658, and O-6668 in CB1(−/−) and CB1(+/+) mice (open and filled bars, respectively) on locomotor activity expressed as number of photocell beam breaks (top), percentage of ring immobility (middle), and change in rectal temperature (bottom). Each bar represents the mean (± S.E.M.) of three to six mice, with the exception that n = 15 to 19 for each genotype in the vehicle condition and n = 2 for ring immobility measure for O-6658 in CB1(+/+) mice. # indicates significant (p < 0.05) main effect of compound in both genotypes compared with vehicle (Veh). * indicates significant interaction, with post hoc difference in effect of compound between genotypes.
Similar articles
- Sphingosine and its analog, the immunosuppressant 2-amino-2-(2-[4-octylphenyl]ethyl)-1,3-propanediol, interact with the CB1 cannabinoid receptor.
Paugh SW, Cassidy MP, He H, Milstien S, Sim-Selley LJ, Spiegel S, Selley DE. Paugh SW, et al. Mol Pharmacol. 2006 Jul;70(1):41-50. doi: 10.1124/mol.105.020552. Epub 2006 Mar 29. Mol Pharmacol. 2006. PMID: 16571654 - Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by delta9-tetrahydrocannabinol and CP55,940 in the rat.
Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Craft RM, et al. J Pharmacol Exp Ther. 2012 Mar;340(3):787-800. doi: 10.1124/jpet.111.188540. Epub 2011 Dec 19. J Pharmacol Exp Ther. 2012. PMID: 22182934 - Pharmacophores for ligand recognition and activation/inactivation of the cannabinoid receptors.
Reggio PH. Reggio PH. Curr Pharm Des. 2003;9(20):1607-33. doi: 10.2174/1381612033454577. Curr Pharm Des. 2003. PMID: 12871061 Review. - Are cannabidiol and Δ(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. McPartland JM, et al. Br J Pharmacol. 2015 Feb;172(3):737-53. doi: 10.1111/bph.12944. Br J Pharmacol. 2015. PMID: 25257544 Free PMC article. Review.
Cited by
- Novel 3-substituted rimonabant analogues lack Δ(9) -tetrahydrocannabinol-like abuse-related behavioural effects in mice.
Walentiny D, Vann R, Mahadevan A, Kottani R, Gujjar R, Wiley J. Walentiny D, et al. Br J Pharmacol. 2013 May;169(1):10-20. doi: 10.1111/bph.12099. Br J Pharmacol. 2013. PMID: 23297801 Free PMC article. - The biarylpyrazole compound AM251 alters mitochondrial physiology via proteolytic degradation of ERRα.
Krzysik-Walker SM, González-Mariscal I, Scheibye-Knudsen M, Indig FE, Bernier M. Krzysik-Walker SM, et al. Mol Pharmacol. 2013 Jan;83(1):157-66. doi: 10.1124/mol.112.082651. Epub 2012 Oct 12. Mol Pharmacol. 2013. PMID: 23066093 Free PMC article. - Structural analogs of pyrazole and sulfonamide cannabinoids: effects on acute food intake in mice.
Wiley JL, Marusich JA, Zhang Y, Fulp A, Maitra R, Thomas BF, Mahadevan A. Wiley JL, et al. Eur J Pharmacol. 2012 Nov 15;695(1-3):62-70. doi: 10.1016/j.ejphar.2012.08.019. Epub 2012 Sep 6. Eur J Pharmacol. 2012. PMID: 22975289 Free PMC article. - Cannabinoids in disguise: Δ9-tetrahydrocannabinol-like effects of tetramethylcyclopropyl ketone indoles.
Wiley JL, Marusich JA, Lefever TW, Grabenauer M, Moore KN, Thomas BF. Wiley JL, et al. Neuropharmacology. 2013 Dec;75:145-54. doi: 10.1016/j.neuropharm.2013.07.022. Epub 2013 Aug 2. Neuropharmacology. 2013. PMID: 23916483 Free PMC article. - Structure-Based Identification of Potent Natural Product Chemotypes as Cannabinoid Receptor 1 Inverse Agonists.
Pandey P, Roy KK, Liu H, Ma G, Pettaway S, Alsharif WF, Gadepalli RS, Rimoldi JM, McCurdy CR, Cutler SJ, Doerksen RJ. Pandey P, et al. Molecules. 2018 Oct 13;23(10):2630. doi: 10.3390/molecules23102630. Molecules. 2018. PMID: 30322136 Free PMC article.
References
- Adams IB, Compton DR, Martin BR. (1998) Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther 284:1209–1217 - PubMed
- Bass CE, Griffin G, Grier M, Mahadevan A, Razdan RK, Martin BR. (2002) SR-141716A-induced stimulation of locomotor activity. A structure-activity relationship study. Pharmacol Biochem Behav 74:31–40 - PubMed
- Breivogel CS, Childers SR. (2000) Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther 295:328–336 - PubMed
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) on an enzymatic reaction. Biochem Pharmacol 22:3099–3108 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA005488/DA/NIDA NIH HHS/United States
- DA-005488/DA/NIDA NIH HHS/United States
- R01 DA003672/DA/NIDA NIH HHS/United States
- DA-03672/DA/NIDA NIH HHS/United States
- R01 DA003672-29/DA/NIDA NIH HHS/United States
- R37 DA003672/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous