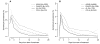A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial - PubMed (original) (raw)
Randomized Controlled Trial
A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial
Four Artemisinin-Based Combinations (4ABC) Study Group. PLoS Med. 2011 Nov.
Abstract
Background: Artemisinin-based combination therapies (ACTs) are the mainstay for the management of uncomplicated malaria cases. However, up-to-date data able to assist sub-Saharan African countries formulating appropriate antimalarial drug policies are scarce.
Methods and findings: Between 9 July 2007 and 19 June 2009, a randomized, non-inferiority (10% difference threshold in efficacy at day 28) clinical trial was carried out at 12 sites in seven sub-Saharan African countries. Each site compared three of four ACTs, namely amodiaquine-artesunate (ASAQ), dihydroartemisinin-piperaquine (DHAPQ), artemether-lumefantrine (AL), or chlorproguanil-dapsone-artesunate (CD+A). Overall, 4,116 children 6-59 mo old with uncomplicated Plasmodium falciparum malaria were treated (1,226 with AL, 1,002 with ASAQ, 413 with CD+A, and 1,475 with DHAPQ), actively followed up until day 28, and then passively followed up for the next 6 mo. At day 28, for the PCR-adjusted efficacy, non-inferiority was established for three pair-wise comparisons: DHAPQ (97.3%) versus AL (95.5%) (odds ratio [OR]: 0.59, 95% CI: 0.37-0.94); DHAPQ (97.6%) versus ASAQ (96.8%) (OR: 0.74, 95% CI: 0.41-1.34), and ASAQ (97.1%) versus AL (94.4%) (OR: 0.50, 95% CI: 0.28-0.92). For the PCR-unadjusted efficacy, AL was significantly less efficacious than DHAPQ (72.7% versus 89.5%) (OR: 0.27, 95% CI: 0.21-0.34) and ASAQ (66.2% versus 80.4%) (OR: 0.40, 95% CI: 0.30-0.53), while DHAPQ (92.2%) had higher efficacy than ASAQ (80.8%) but non-inferiority could not be excluded (OR: 0.35, 95% CI: 0.26-0.48). CD+A was significantly less efficacious than the other three treatments. Day 63 results were similar to those observed at day 28.
Conclusions: This large head-to-head comparison of most currently available ACTs in sub-Saharan Africa showed that AL, ASAQ, and DHAPQ had excellent efficacy, up to day 63 post-treatment. The risk of recurrent infections was significantly lower for DHAPQ, followed by ASAQ and then AL, supporting the recent recommendation of considering DHAPQ as a valid option for the treatment of uncomplicated P. falciparum malaria.
Trial registration: ClinicalTrials.gov NCT00393679; Pan African Clinical Trials Registry PACTR2009010000911750
Conflict of interest statement
Prof. Umberto d‚Alessandro has received research funding from Sigma Tau and Sanofi-Aventis. Dr. Quique Bassat has received in the past three years speaker fees and travel grants from Sigma Tau SP, Pomezia, Rome, Italy. All other authors declare no competing interests.
Figures
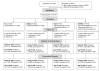
Figure 1. Trial profile up to day 28.
Adj TF, adjusted treatment failure; Unadj TF, unadjusted treatment failure.
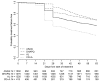
Figure 2. Proportion of patients whose treatment was failure-free by day of follow-up.
ITT population; data pooled over all sites.
Figure 3. Six pair-wise comparisons at day 28 and day 63.
OR (circles), 95% CI (horizontal bars), and non-inferiority limit (vertical bars) for each pair-wise analysis for PCR-unadjusted (left panel) and -adjusted (right panel) ACPR at days 28 (filled circles) and 63 (open circles) (ITT population). An OR and 95% CI<1 indicate a significantly higher efficacy of the second versus the first treatment of the pair-wise comparison. The non-inferiority limits (day 28 only) correspond to a 10% difference in efficacy, recalculated to an OR scale. Non-inferiority is established if the 95% CI lies completely above the non-inferiority limit.
Figure 4. Treatment efficacy by pair-wise comparison and by site.
Risk difference and 95% CI for three pair-wise analyses by site for PCR-unadjusted (left panel) and -adjusted (right panel) ACPR at days 28 (filled circles) and 63 (open circles) (ITT population) (A) ASAQ versus DHAPQ; (B) AL versus ASAQ; (C) AL versus DHAPQ. BF, Burkina Faso; GA, Gabon; MZ, Mozambique; NG, Nigeria; RW, Rwanda; UG, Uganda; ZM, Zimbabwe.
Figure 5. Gametocyte prevalence by treatment and day of follow-up.
(A) All patients regardless of gametocytemia at enrollment; (B) patients with gametocytes at enrollment excluded.
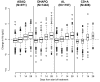
Figure 6. Hematological recovery by treatment and day of follow-up.
Hematological recovery determined by Hb changes compared to day 0. The boxplots contain the following information: median (bold line), first and third quartile (box), whiskers extending to 1.5× interquartile range, and all more extreme values.
Similar articles
- [Evaluation of the efficacy and safety of three 2-drug combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Senegal: artesunate-amodiaquine, dihydroartemisinin-piperaquine, and artemether-lumefantrine].
Sow D, Ndiaye JL, Sylla K, Ba MS, Tine RC, Faye B, Pene M, Ndiaye M, Seck A, Lo AC, Abiola A, Dieng Y, Gaye O. Sow D, et al. Med Sante Trop. 2016 Jan-Mar;26(1):45-50. doi: 10.1684/mst.2015.0524. Med Sante Trop. 2016. PMID: 26644184 Clinical Trial. French. - Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial.
West African Network for Clinical Trials of Antimalarial Drugs (WANECAM). West African Network for Clinical Trials of Antimalarial Drugs (WANECAM). Lancet. 2018 Apr 7;391(10128):1378-1390. doi: 10.1016/S0140-6736(18)30291-5. Epub 2018 Mar 29. Lancet. 2018. PMID: 29606364 Free PMC article. Clinical Trial. - Randomized non-inferiority and safety trial of dihydroartemisin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children.
Nji AM, Ali IM, Moyeh MN, Ngongang EO, Ekollo AM, Chedjou JP, Ndikum VN, Evehe MS, Froeschl G, Heumann C, Mansmann U, Ogundahunsi O, Mbacham WF. Nji AM, et al. Malar J. 2015 Jan 28;14:27. doi: 10.1186/s12936-014-0521-2. Malar J. 2015. PMID: 25626448 Free PMC article. Clinical Trial. - Therapeutic efficacy of artemether-lumefantrine, artesunate-amodiaquine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Sub-Saharan Africa: A systematic review and meta-analysis.
Marwa K, Kapesa A, Baraka V, Konje E, Kidenya B, Mukonzo J, Kamugisha E, Swedberg G. Marwa K, et al. PLoS One. 2022 Mar 10;17(3):e0264339. doi: 10.1371/journal.pone.0264339. eCollection 2022. PLoS One. 2022. PMID: 35271592 Free PMC article. - Dihydroartemisinin-piperaquine for treating uncomplicated Plasmodium falciparum malaria.
Zani B, Gathu M, Donegan S, Olliaro PL, Sinclair D. Zani B, et al. Cochrane Database Syst Rev. 2014 Jan 20;2014(1):CD010927. doi: 10.1002/14651858.CD010927. Cochrane Database Syst Rev. 2014. PMID: 24443033 Free PMC article. Review.
Cited by
- Repeat polymorphisms in the low-complexity regions of Plasmodium falciparum ABC transporters and associations with in vitro antimalarial responses.
Okombo J, Abdi AI, Kiara SM, Mwai L, Pole L, Sutherland CJ, Nzila A, Ochola-Oyier LI. Okombo J, et al. Antimicrob Agents Chemother. 2013 Dec;57(12):6196-204. doi: 10.1128/AAC.01465-13. Epub 2013 Sep 30. Antimicrob Agents Chemother. 2013. PMID: 24080667 Free PMC article. - Clinical Implications of Asymptomatic Plasmodium falciparum Infections in Malawi.
Buchwald AG, Sixpence A, Chimenya M, Damson M, Sorkin JD, Wilson ML, Seydel K, Hochman S, Mathanga DP, Taylor TE, Laufer MK. Buchwald AG, et al. Clin Infect Dis. 2019 Jan 1;68(1):106-112. doi: 10.1093/cid/ciy427. Clin Infect Dis. 2019. PMID: 29788054 Free PMC article. - Antimalarial Resistance Unlikely To Explain U.K. Artemether-Lumefantrine Failures.
van der Pluijm RW, Watson J, Woodrow CJ. van der Pluijm RW, et al. Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00721-17. doi: 10.1128/AAC.00721-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28655744 Free PMC article. No abstract available. - Demography, maternal health and the epidemiology of malaria and other major infectious diseases in the rural department Tsamba-Magotsi, Ngounie Province, in central African Gabon.
Manego RZ, Mombo-Ngoma G, Witte M, Held J, Gmeiner M, Gebru T, Tazemda B, Mischlinger J, Groger M, Lell B, Adegnika AA, Agnandji ST, Kremsner PG, Mordmüller B, Ramharter M, Matsiegui PB. Manego RZ, et al. BMC Public Health. 2017 Jan 28;17(1):130. doi: 10.1186/s12889-017-4045-x. BMC Public Health. 2017. PMID: 28129759 Free PMC article. - Dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria during pregnancy and risk of malaria in early childhood: A randomized controlled trial.
Jagannathan P, Kakuru A, Okiring J, Muhindo MK, Natureeba P, Nakalembe M, Opira B, Olwoch P, Nankya F, Ssewanyana I, Tetteh K, Drakeley C, Beeson J, Reiling L, Clark TD, Rodriguez-Barraquer I, Greenhouse B, Wallender E, Aweeka F, Prahl M, Charlebois ED, Feeney ME, Havlir DV, Kamya MR, Dorsey G. Jagannathan P, et al. PLoS Med. 2018 Jul 17;15(7):e1002606. doi: 10.1371/journal.pmed.1002606. eCollection 2018 Jul. PLoS Med. 2018. PMID: 30016328 Free PMC article. Clinical Trial.
References
- Prudhomme O'Meara W, Nekesa Mangeni J, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. - PubMed
- World Health Organization. World malaria report 2009. Geneva: World Health Organization; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources