Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial - PubMed (original) (raw)
Clinical Trial
. 2011 Nov 26;378(9806):1847-57.
doi: 10.1016/S0140-6736(11)61590-0. Epub 2011 Nov 14.
Atul R Chugh, Domenico D'Amario, John H Loughran, Marcus F Stoddard, Sohail Ikram, Garth M Beache, Stephen G Wagner, Annarosa Leri, Toru Hosoda, Fumihiro Sanada, Julius B Elmore, Polina Goichberg, Donato Cappetta, Naresh K Solankhi, Ibrahim Fahsah, D Gregg Rokosh, Mark S Slaughter, Jan Kajstura, Piero Anversa
Affiliations
- PMID: 22088800
- PMCID: PMC3614010
- DOI: 10.1016/S0140-6736(11)61590-0
Clinical Trial
Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial
Roberto Bolli et al. Lancet. 2011.
Retraction in
- Retraction-Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial.
The Lancet Editors. The Lancet Editors. Lancet. 2019 Mar 16;393(10176):1084. doi: 10.1016/S0140-6736(19)30542-2. Epub 2019 Mar 14. Lancet. 2019. PMID: 30894259 Free PMC article. No abstract available.
Expression of concern in
- Expression of concern: the SCIPIO trial.
The Lancet Editors. The Lancet Editors. Lancet. 2014 Apr 12;383(9925):1279. doi: 10.1016/S0140-6736(14)60608-5. Lancet. 2014. PMID: 24725564 Free PMC article. No abstract available.
Abstract
Background: c-kit-positive, lineage-negative cardiac stem cells (CSCs) improve post-infarction left ventricular (LV) dysfunction when administered to animals. We undertook a phase 1 trial (Stem Cell Infusion in Patients with Ischemic cardiOmyopathy [SCIPIO]) of autologous CSCs for the treatment of heart failure resulting from ischaemic heart disease.
Methods: In stage A of the SCIPIO trial, patients with post-infarction LV dysfunction (ejection fraction [EF] ≤40%) before coronary artery bypass grafting were consecutively enrolled in the treatment and control groups. In stage B, patients were randomly assigned to the treatment or control group in a 2:3 ratio by use of a computer-generated block randomisation scheme. 1 million autologous CSCs were administered by intracoronary infusion at a mean of 113 days (SE 4) after surgery; controls were not given any treatment. Although the study was open label, the echocardiographic analyses were masked to group assignment. The primary endpoint was short-term safety of CSCs and the secondary endpoint was efficacy. A per-protocol analysis was used. This study is registered with ClinicalTrials.gov, number NCT00474461.
Findings: This study is still in progress. 16 patients were assigned to the treatment group and seven to the control group; no CSC-related adverse effects were reported. In 14 CSC-treated patients who were analysed, LVEF increased from 30·3% (SE 1·9) before CSC infusion to 38·5% (2·8) at 4 months after infusion (p=0·001). By contrast, in seven control patients, during the corresponding time interval, LVEF did not change (30·1% [2·4] at 4 months after CABG vs 30·2% [2·5] at 8 months after CABG). Importantly, the salubrious effects of CSCs were even more pronounced at 1 year in eight patients (eg, LVEF increased by 12·3 ejection fraction units [2·1] vs baseline, p=0·0007). In the seven treated patients in whom cardiac MRI could be done, infarct size decreased from 32·6 g (6·3) by 7·8 g (1·7; 24%) at 4 months (p=0·004) and 9·8 g (3·5; 30%) at 1 year (p=0·04).
Interpretation: These initial results in patients are very encouraging. They suggest that intracoronary infusion of autologous CSCs is effective in improving LV systolic function and reducing infarct size in patients with heart failure after myocardial infarction, and warrant further, larger, phase 2 studies.
Funding: University of Louisville Research Foundation and National Institutes of Health.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of Interest. PA is a member of Autologous, LLC. The other authors declare that they have no conflicts of interest.
Figures
Figure 1
Trial profile showing a summary of screening, exclusions, and enrollment. Patients were screened at two time points: during the 2 weeks preceding CABG surgery (initial eligibility screening and enrollment), and at 4 ± 1 months after CABG surgery (final enrollment). The trial consisted of two sequential Stages, A (non-randomized) and B (randomized). To assess feasibility and short-term safety of CSC therapy, in Stage A nine consecutive patients were assigned to the treatment arm followed by assignment of four consecutive patients to the control arm. Then, in Stage B, patients were randomized to the treated and control arms in a 2:3 ratio using a block randomization scheme and a block size of five. The Figure summarizes enrollment as of April 1, 2011.
Figure 2
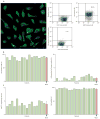
Phenotype of CSCs prior to intracoronary delivery. A, confocal image illustrating the localization of c-kit (green) in CSCs and the FACS analysis of c-kit expression and lineage markers of CSCs for Subject 019. Numerical values are indicated. B, percentage of cells expressing c-kit, lineage markers of cardiac commitment, and the senescence-associated protein p16INK4a. The percentage of viable cells in the final preparation is also illustrated. Yellow bars indicate individual values, red bars indicate means ± SEM.
Figure 3
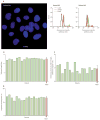
Growth properties of CSCs prior to intracoronary delivery. A, telomeres in CSC nuclei (red dots) are identified by Q-FISH (left panel, Subject 019) and flow-FISH (central and right panels, Subjects 055 and 056). R cells with long telomeres (48 kbp) and S cells with short telomeres (7 kbp) were used to compute absolute values. In each panel, telomere length is indicated. By flow-FISH, the histograms represent the intensity of PNA probe binding in gated CSCs (red) and control cells (green). B, telomerase activity in CSC lysates from each patient was detected by quantitative PCR. C, population doubling time in CSCs. Yellow bars indicate individual values, red bars indicate means ± SEM.
Figure 4
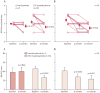
Echocardiographic analysis at 4 and 12 months after baseline (baseline was 4 ± 1 month after surgical revascularization). A, left ventricular ejection fraction (EF) (measured by 3D echocardiography) at 4 months after baseline in control and treated patients. B, EF at 4 and 12 months after baseline in the eight treated patients that have 1 year of follow-up. C, change in EF from baseline at 4 and 12 months in treated patients (absolute EF units). D, wall motion score index (WMSI) at 4 months after baseline in control and treated patients. E, WMSI at 4 and 12 months after baseline in the eight treated patients that have 1 year of follow-up. Data are means ± SEM.
Figure 5
Cardiac magnetic resonance (cMR) analysis at 4 and 12 months after baseline in treated patients (baseline was 4 ± 1 month after surgical revascularization). A, infarct size. B, change in infarct size from baseline. Data are means ± SEM.
Figure 6
Functional status/quality of life. New York Heart Association (NYHA) functional class (A and B) and quality of life (evaluated by the Minnesota Living with Heart Failure Questionnaire [MLHFQ] score) (C and D) at 4 and 12 months after baseline (baseline was 4 ± 1 month after surgical revascularization). A, NYHA functional class and C, MLHFQ score at 4 months after baseline in control and treated patients. B, NYHA functional class and D, MLHFQ score at 4 and 12 months after baseline in the 10 treated patients that have 1 year of follow-up. Data are means ± SEM.
Comment in
- SCIPIO brings new momentum to cardiac cell therapy.
Heusch G. Heusch G. Lancet. 2011 Nov 26;378(9806):1827-8. doi: 10.1016/S0140-6736(11)61648-6. Epub 2011 Nov 14. Lancet. 2011. PMID: 22088799 No abstract available. - Cardiac stem cells in patients with ischaemic cardiomyopathy.
Stamm C, Nasseri B, Hetzer R. Stamm C, et al. Lancet. 2012 Mar 10;379(9819):891. doi: 10.1016/S0140-6736(12)60385-7. Lancet. 2012. PMID: 22405788 No abstract available. - Cardiac stem cells in patients with ischaemic cardiomyopathy.
Masuda S. Masuda S. Lancet. 2012 Mar 10;379(9819):891. doi: 10.1016/S0140-6736(12)60386-9. Lancet. 2012. PMID: 22405790 No abstract available.
Similar articles
- Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance.
Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Chugh AR, et al. Circulation. 2012 Sep 11;126(11 Suppl 1):S54-64. doi: 10.1161/CIRCULATIONAHA.112.092627. Circulation. 2012. PMID: 22965994 Free PMC article. Clinical Trial. - Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial.
Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Makkar RR, et al. Lancet. 2012 Mar 10;379(9819):895-904. doi: 10.1016/S0140-6736(12)60195-0. Epub 2012 Feb 14. Lancet. 2012. PMID: 22336189 Free PMC article. Clinical Trial. - Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy.
Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Bolli R, et al. Circulation. 2013 Jul 9;128(2):122-31. doi: 10.1161/CIRCULATIONAHA.112.001075. Epub 2013 Jun 11. Circulation. 2013. PMID: 23757309 Free PMC article. - Stem cell therapy for chronic ischaemic heart disease and congestive heart failure.
Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Fisher SA, et al. Cochrane Database Syst Rev. 2014 Apr 29;(4):CD007888. doi: 10.1002/14651858.CD007888.pub2. Cochrane Database Syst Rev. 2014. PMID: 24777540 Updated. Review.
Cited by
- Intracellular aggregation of multimodal silica nanoparticles for ultrasound-guided stem cell implantation.
Jokerst JV, Khademi C, Gambhir SS. Jokerst JV, et al. Sci Transl Med. 2013 Mar 20;5(177):177ra35. doi: 10.1126/scitranslmed.3005228. Sci Transl Med. 2013. PMID: 23515077 Free PMC article. - Bioenergetic and functional consequences of cellular therapy: activation of endogenous cardiovascular progenitor cells.
Xiong Q, Ye L, Zhang P, Lepley M, Swingen C, Zhang L, Kaufman DS, Zhang J. Xiong Q, et al. Circ Res. 2012 Aug 3;111(4):455-68. doi: 10.1161/CIRCRESAHA.112.269894. Epub 2012 Jun 21. Circ Res. 2012. PMID: 22723295 Free PMC article. - Safe genetic modification of cardiac stem cells using a site-specific integration technique.
Lan F, Liu J, Narsinh KH, Hu S, Han L, Lee AS, Karow M, Nguyen PK, Nag D, Calos MP, Robbins RC, Wu JC. Lan F, et al. Circulation. 2012 Sep 11;126(11 Suppl 1):S20-8. doi: 10.1161/CIRCULATIONAHA.111.084913. Circulation. 2012. PMID: 22965984 Free PMC article. - Developing miRNA therapeutics for cardiac repair in ischemic heart disease.
Zhu K, Liu D, Lai H, Li J, Wang C. Zhu K, et al. J Thorac Dis. 2016 Sep;8(9):E918-E927. doi: 10.21037/jtd.2016.08.93. J Thorac Dis. 2016. PMID: 27747027 Free PMC article. Review. - Functional screening identifies miRNAs inducing cardiac regeneration.
Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Eulalio A, et al. Nature. 2012 Dec 20;492(7429):376-81. doi: 10.1038/nature11739. Epub 2012 Dec 5. Nature. 2012. PMID: 23222520
References
- Coronary Heart Disease Statistics. British Heart Foundation; 2003.
- Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV. More malignant than cancer? Five year hospitalization after a first hospitalization with heart failure. Eur Heart Fail. 2001;3:315–22. - PubMed
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch intern Med. 2007;167(10):989–97. - PubMed
- Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114(6):763–76. - PubMed
Publication types
MeSH terms
Grants and funding
- R37 HL081737-01/HL/NHLBI NIH HHS/United States
- P01 HL078825/HL/NHLBI NIH HHS/United States
- R01 HL039902/HL/NHLBI NIH HHS/United States
- R37 HL081737/HL/NHLBI NIH HHS/United States
- R37HL081737/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical