Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases - PubMed (original) (raw)
Review
Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases
Mariola J Edelmann et al. Expert Rev Mol Med. 2011.
Free PMC article
Abstract
Recent advances in the development and discovery of pharmacological interventions within the ubiquitin-proteasome system (UPS) have uncovered an enormous potential for possible novel treatments of neurodegenerative disease, cancer, immunological disorder and microbial infection. Interference with proteasome activity, although initially considered unlikely to be exploitable clinically, has already proved to be very effective against haematological malignancies, and more specific derivatives that target subsets of proteasomes are emerging. Recent small-molecule screens have revealed inhibitors against ubiquitin-conjugating and -deconjugating enzymes, many of which have been evaluated for their potential use as therapeutics, either as single agents or in synergy with other drugs. Here, we discuss recent advances in the characterisation of novel UPS modulators (in particular, inhibitors of ubiquitin-conjugating and -deconjugating enzymes) and how they pave the way towards new therapeutic approaches for the treatment of proteotoxic disease, cancer and microbial infection.
Figures
Figure 1
Small-molecule inhibitors in the ubiquitin–proteasome system (UPS). Schematic representation of components of the UPS including E1, E2–E3 ligases, DUBs and the proteasome complex (20Si: immunoproteasome). Ubiquitin is indicated as pink circle labelled U. The UPS pathway and different examples of E1, E2, E3s and DUBs are highlighted in blue boxes. Increasing numbers of small-molecule inhibitors that interfere at various steps of the UPS cascade are being discovered.
Figure 2
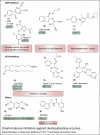
Small-molecule inhibitors against deubiquitylating enzymes. Examples of DUB inhibitors characterised in the literature targeting the USP family: HBX41,108 (1) (Ref. 50) and P022077 (2) (Ref. 51) specific for USP7, HBX 90,397 inhibits USP8 (3) (Ref. 52) and IU1 (7) inhibits USP14 (Ref. 53). Inhibitors targeting the UCH family include 15d-PGJ2 (Ref. 54) (4) and isatin _O_-acyl oximes (5) (Ref. 55) specific against UCH-L3 and other isatin derivatives (6) specific against UCH-L1 (Refs 56, 57). PR-619 (6) targets a broad range of DUBs (Ref. 51) and GRL0617 (9) inhibits SARS virus encoded papain-like protease (PLpro) (Ref. 58).
Figure 3
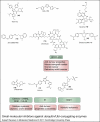
Small-molecule inhibitors against ubiquitin/Ubl-conjugating enzymes. Examples of inhibitors against E3 ligases include Nutlin-3a (10), RITA (11), MI-219 (12), JNJ-26854165 (13), chlorofusin (14) and chalcone (AM114) (15), which specifically interfere with the HDM2–p53 or HDM2–proteasome interactions (Refs 88, 89, 90, 91, 92, 93), and thalidomide (16), which inhibits CRBN (Ref. 94). The ubiquitin-activating enzyme E1 is targeted by PYR-41 (Ref. 95) (17), and the NEDD8-activating enzyme NAE1 is inhibited by MLN4924 (18) (Ref. 96). All molecular targets are associated with disease pathologies, in particular cancer. See text for further details. Abbreviations: CRBN, cereblon; NEDD8, neural precursor cell expressed developmentally down-regulated 8; Ubl, ubiquitin-like protein.
Similar articles
- Emerging role of the ubiquitin-proteasome system as drug targets.
Kar G, Keskin O, Fraternali F, Gursoy A. Kar G, et al. Curr Pharm Des. 2013;19(18):3175-89. doi: 10.2174/1381612811319180002. Curr Pharm Des. 2013. PMID: 23151131 Review. - Ubiquitin becomes ubiquitous in cancer: emerging roles of ubiquitin ligases and deubiquitinases in tumorigenesis and as therapeutic targets.
Shi D, Grossman SR. Shi D, et al. Cancer Biol Ther. 2010 Oct 15;10(8):737-47. doi: 10.4161/cbt.10.8.13417. Epub 2010 Oct 15. Cancer Biol Ther. 2010. PMID: 20930542 Free PMC article. Review. - Drug discovery in the ubiquitin-proteasome system.
Nalepa G, Rolfe M, Harper JW. Nalepa G, et al. Nat Rev Drug Discov. 2006 Jul;5(7):596-613. doi: 10.1038/nrd2056. Nat Rev Drug Discov. 2006. PMID: 16816840 Review. - Targeting the ubiquitin proteasome system: beyond proteasome inhibition.
Xolalpa W, Perez-Galan P, Rodríguez MS, Roué G. Xolalpa W, et al. Curr Pharm Des. 2013;19(22):4053-93. doi: 10.2174/1381612811319220014. Curr Pharm Des. 2013. PMID: 23181575 Review. - Unstructured Biology of Proteins from Ubiquitin-Proteasome System: Roles in Cancer and Neurodegenerative Diseases.
Gadhave K, Kumar P, Kapuganti SK, Uversky VN, Giri R. Gadhave K, et al. Biomolecules. 2020 May 21;10(5):796. doi: 10.3390/biom10050796. Biomolecules. 2020. PMID: 32455657 Free PMC article.
Cited by
- Direct measurement of deubiquitinating enzyme activity in intact cells using a protease-resistant, cell-permeable, peptide-based reporter.
Safa N, Pettigrew JH, Gauthier TJ, Melvin AT. Safa N, et al. Biochem Eng J. 2019 Nov 15;151:107320. doi: 10.1016/j.bej.2019.107320. Epub 2019 Jul 29. Biochem Eng J. 2019. PMID: 32831622 Free PMC article. - Sarcoidosis activates diverse transcriptional programs in bronchoalveolar lavage cells.
Gharib SA, Malur A, Huizar I, Barna BP, Kavuru MS, Schnapp LM, Thomassen MJ. Gharib SA, et al. Respir Res. 2016 Jul 26;17(1):93. doi: 10.1186/s12931-016-0411-y. Respir Res. 2016. PMID: 27460362 Free PMC article. - The Ubiquitin System: An Emerging Therapeutic Target for Lung Cancer.
Jin JO, Puranik N, Bui QT, Yadav D, Lee PC. Jin JO, et al. Int J Mol Sci. 2021 Sep 6;22(17):9629. doi: 10.3390/ijms22179629. Int J Mol Sci. 2021. PMID: 34502538 Free PMC article. Review. - Synthesis and in vitro anticancer evaluation of some 4,6-diamino-1,3,5-triazine-2-carbohydrazides as Rad6 ubiquitin conjugating enzyme inhibitors.
Kothayer H, Spencer SM, Tripathi K, Westwell AD, Palle K. Kothayer H, et al. Bioorg Med Chem Lett. 2016 Apr 15;26(8):2030-4. doi: 10.1016/j.bmcl.2016.02.085. Epub 2016 Mar 2. Bioorg Med Chem Lett. 2016. PMID: 26965855 Free PMC article. - Emerging role of DUBs in tumor metastasis and apoptosis: Therapeutic implication.
He M, Zhou Z, Wu G, Chen Q, Wan Y. He M, et al. Pharmacol Ther. 2017 Sep;177:96-107. doi: 10.1016/j.pharmthera.2017.03.001. Epub 2017 Mar 6. Pharmacol Ther. 2017. PMID: 28279784 Free PMC article. Review.
References
- Behrends C., Harper J.W.. Constructing and decoding unconventional ubiquitin chains. Nature Structural and Molecular Biology. 2011;18:520–528. - PubMed
- Clague M.J., Urbe S.. Ubiquitin: same molecule, different degradation pathways. Cell. 2010;143:682–685. - PubMed
- Eldridge A.G., O'Brien T.. Therapeutic strategies within the ubiquitin proteasome system. Cell Death and Differentiation. 2010;17:4–13. - PubMed
Further reading, resources and contacts
- Nalepa G., Rolfe M., Harper J.W.. Drug discovery in the ubiquitin–proteasome system. Nature Reviews. Drug Discovery. 2006;5:596–613. - PubMed
- Eldridge A.G., O'Brien T.. Therapeutic strategies within the ubiquitin proteasome system. Cell Death and Differentiation. 2010;17:4–13. - PubMed
- Dickens M.P., Fitzgerald R., Fischer P.M.. Small-molecule inhibitors of MDM2 as new anticancer therapeutics. Seminars in Cancer Biology. 2010;20:10–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R43 CA115205/CA/NCI NIH HHS/United States
- CA115205/CA/NCI NIH HHS/United States
- R44 CA115205/CA/NCI NIH HHS/United States
- G0501068/MRC_/Medical Research Council/United Kingdom
- DK07139/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical