Linking neurodevelopmental and synaptic theories of mental illness through DISC1 - PubMed (original) (raw)
Review
Linking neurodevelopmental and synaptic theories of mental illness through DISC1
Nicholas J Brandon et al. Nat Rev Neurosci. 2011.
Abstract
Recent advances in our understanding of the underlying genetic architecture of psychiatric disorders has blown away the diagnostic boundaries that are defined by currently used diagnostic manuals. The disrupted in schizophrenia 1 (DISC1) gene was originally discovered at the breakpoint of an inherited chromosomal translocation, which segregates with major mental illnesses. In addition, many biological studies have indicated a role for DISC1 in early neurodevelopment and synaptic regulation. Given that DISC1 is thought to drive a range of endophenotypes that underlie major mental conditions, elucidating the biology of DISC1 may enable the construction of new diagnostic categories for mental illnesses with a more meaningful biological foundation.
Figures
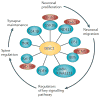
Figure 1. The ‘DISC1 interactome’ points towards the multiple functions of DISC1 during neuronal development
The important DISC1 interactions relevant for this review are highlighted in this figure. They are a small subset of the original dataset, which have all been confirmed in other biological systems,,,,,,,,. The functional relevance of the different interactions is highlighted on the perimeter of the cartoon, with arrows representing the chronological order of events during development. DISC1, Disrupted-in-schizophrenia-1; GSK3β, Glycogen synthase kinase 3β; Dixdc1, DIX domain containing 1; NDEL1, Nuclear distribution protein nudE-like 1; LIS1, Lissencephaly protein 1; PCM1, Pericentriolar material 1; BBS, Bardet-Biedl Syndrome protein; PDE4; Phosphodiesterase type 4; Kal-7, Kalirin-7; TNIK, TRAF2 and NCK-interacting protein kinase; and PSD95; Postsynaptic density protein 95.
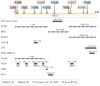
Figure 2. DISC1 protein interaction domains and relationship to the location of rare human variants associated with mental disorders
Diagram of protein-protein interaction domains mapped onto DISC1 and key DISC1 genetic variants (top) possibly associated with mental disorders–,,,,,,,,,,. PCM1, Pericentriolar material 1; BBS, Bardet-Biedl Syndrome; Dixdc1, DIX domain containing 1; GSK3β Glycogen synthase kinase 3β; LIS1, Lissencephaly protein 1; NDE1, Nuclear Distribution protein NudE homolog 1; NDEL1, Nuclear Distribution Protein NudE-Like 1; PDE4, Phosphodiesterase type 4; TNIK, TRAF2 and NCK-interacting protein kinase; and Kal-7, Kalirin-7. Genetic variants at Q264R, L607F, and S704C (common variants) are associated with schizophrenia (SZ). Furthermore, associations with L607F (*) and S704C (#) are reported for schizoaffective (SA) and major depression (MD), respectively. BP, bipolar disorder. Please note that the binding sites have been mapped using a range of different approaches so the resolution achieved in individual interactions is different (dotted lines are sites awaiting fine mapping).
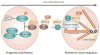
Figure 3. DISC1 function during early development
DISC1 regulates both progenitor cell proliferation and postmitotic neuronal migration. In proliferating progenitors, DISC1 inhibits premature cell cycle exit and differentiation of proliferating progenitors: In these cells, DISC1 inhibits GSK3β through direct physical interaction, which in turn reduces β-catenin phosphorylation and stabilizes β-catenin. Green circles with the black and white stripe indicate the rapid degradation of this protein. In postmitotic neurons DISC1 preferentially interacts with proteins in the dynein motor complexes associated with microtubules (pink tubes) and the centrosome (two grey connected ovals), including BBS proteins, which mediates neuronal migration,. As corticogenesis progresses, the affinity of DISC1 for GSK3β is reduced, likely through phosphorylation at S713; in contrast, the interaction with BBS proteins is increased. Furthermore, DISC1 regulates the transition from neuronal progenitor proliferation to neuronal migration through an increase in phosphorylation of DISC1 at S710, which occurs at embryonic stage E13-15: DISC1/BBS binding recruits DISC1 away from a GSK3β-regulated Wnt/β-catenin activity and thus contributes to the switch from neuronal progenitor proliferation to neuronal migration. DISC1, Disrupted-in-Schizophrenia-1; BBS, Bardet-Biedl Syndrome protein; and GSK3β, Glycogen synthase kinase 3β.
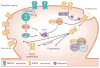
Figure 4. DISC1 function at the synapse
DISC1 has been recently shown to regulate dendritic spines through regulation of a Kal-7-Rac1-PAK pathway and synapse maintenance through regulation of TNIK. DISC1 enhances binding of Kal-7 and PSD95, blocking the access of Kal-7 to Rac1. Once the NMDA receptor is activated, these protein interactions are weakened, allowing the free access of Kal-7 to Rac1, leading to the activation of Rac1. Regulation of Kal-7’s access to Rac1 by DISC1 in an activity-dependent fashion is crucial for the proper maintenance of the synaptic spine. It is currently unknown if TNIK is found in similar or different synapses as DISC1 and kalirin-7 complexes. Inhibition of TNIK function leads to the selective degradation of a number of key postsynaptic proteins including GluR1 and the scaffold protein PSD-95 and a loss of GluR1 from the membrane surface. The degradation of protein is either lysosomal or proteasomal mediated. The E3 ubiquitin ligase, Neuronal precursor cell-expressed developmentally downregulated protein 4-1 (Nedd4-1),, has been identified as a TNIK binding partner. Nedd4-1 has been shown to ubiquitinate GluR1 and drive its lysosomal degradation. Hypothetically, inhibition of TNIK, could lead to an elevated activity of Nedd4-1, increased ubiquitination of GluR1 (or other proteins) and lead to their subsequent degradation. An integrated view of these two pathways is currently unavailable, though both pathways have common end-points related to actin cytoskeleton regulation and AMPA receptor trafficking. Independent studies suggest that TNIK may regulate GluR1 endocytosis through a complex with the E3 ligase Nedd4-1. Complexes of DISC1 with PDE4, GSK3 and NDEL1 (not shown) are also found at the synapse but their function is not known. DISC1, Disrupted-in-Schizophrenia-1; TNIK, TRAF2 and NCK-interacting protein kinase; Nedd4-1, E3 ubiquitin ligase, Neuronal precursor cell-expressed developmentally downregulated protein 4-1; GSK3β, Glycogen synthase kinase 3β; NDEL1, Nuclear distribution protein nudE-like 1; LIS1, Lissencephaly protein 1; PDE4; Phosphodiesterase type 4; Kal-7, Kalirin-7; and PSD95; Postsynaptic density protein 95; NMDAR, N-methyl-D-aspartic acid type glutamate receptor; AMPAR, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionatetyoe glutamate receptor.
Similar articles
- Understanding the role of DISC1 in psychiatric disease and during normal development.
Brandon NJ, Millar JK, Korth C, Sive H, Singh KK, Sawa A. Brandon NJ, et al. J Neurosci. 2009 Oct 14;29(41):12768-75. doi: 10.1523/JNEUROSCI.3355-09.2009. J Neurosci. 2009. PMID: 19828788 Free PMC article. Review. - DISC1 at 10: connecting psychiatric genetics and neuroscience.
Porteous DJ, Millar JK, Brandon NJ, Sawa A. Porteous DJ, et al. Trends Mol Med. 2011 Dec;17(12):699-706. doi: 10.1016/j.molmed.2011.09.002. Epub 2011 Oct 19. Trends Mol Med. 2011. PMID: 22015021 Free PMC article. Review. - The DISC locus in psychiatric illness.
Chubb JE, Bradshaw NJ, Soares DC, Porteous DJ, Millar JK. Chubb JE, et al. Mol Psychiatry. 2008 Jan;13(1):36-64. doi: 10.1038/sj.mp.4002106. Epub 2007 Oct 2. Mol Psychiatry. 2008. PMID: 17912248 Review. - Disrupted in schizophrenia 1 and synaptic function in the mammalian central nervous system.
Randall AD, Kurihara M, Brandon NJ, Brown JT. Randall AD, et al. Eur J Neurosci. 2014 Apr;39(7):1068-73. doi: 10.1111/ejn.12500. Eur J Neurosci. 2014. PMID: 24712987 Free PMC article. Review. - DISC1 and DISC2: discovering and dissecting molecular mechanisms underlying psychiatric illness.
Millar JK, James R, Brandon NJ, Thomson PA. Millar JK, et al. Ann Med. 2004;36(5):367-78. doi: 10.1080/07853890410033603. Ann Med. 2004. PMID: 15478311 Review.
Cited by
- Disrupted-in-schizophrenia (DISC1) functions presynaptically at glutamatergic synapses.
Maher BJ, LoTurco JJ. Maher BJ, et al. PLoS One. 2012;7(3):e34053. doi: 10.1371/journal.pone.0034053. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479520 Free PMC article. - Cognition and Reward Circuits in Schizophrenia: Synergistic, Not Separate.
Robison AJ, Thakkar KN, Diwadkar VA. Robison AJ, et al. Biol Psychiatry. 2020 Feb 1;87(3):204-214. doi: 10.1016/j.biopsych.2019.09.021. Epub 2019 Oct 3. Biol Psychiatry. 2020. PMID: 31733788 Free PMC article. Review. - Adolescent Δ9-Tetrahydrocannabinol Exposure and Astrocyte-Specific Genetic Vulnerability Converge on Nuclear Factor-κB-Cyclooxygenase-2 Signaling to Impair Memory in Adulthood.
Jouroukhin Y, Zhu X, Shevelkin AV, Hasegawa Y, Abazyan B, Saito A, Pevsner J, Kamiya A, Pletnikov MV. Jouroukhin Y, et al. Biol Psychiatry. 2019 Jun 1;85(11):891-903. doi: 10.1016/j.biopsych.2018.07.024. Epub 2018 Aug 16. Biol Psychiatry. 2019. PMID: 30219209 Free PMC article. - Chronic N-Acetylcysteine Treatment Prevents Amphetamine-Induced Hyperactivity in Heterozygous Disc1 Mutant Mice, a Putative Prodromal Schizophrenia Animal Model.
Lai CC, Baskaran R, Tsao CY, Tuan LH, Siow PF, Palani M, Lee LJ, Liu CM, Hwu HG, Lee LJ. Lai CC, et al. Int J Mol Sci. 2022 Aug 20;23(16):9419. doi: 10.3390/ijms23169419. Int J Mol Sci. 2022. PMID: 36012679 Free PMC article. - Disrupted-in-Schizophrenia 1 (DISC1) Overexpression and Juvenile Immune Activation Cause Sex-Specific Schizophrenia-Related Psychopathology in Rats.
Uzuneser TC, Speidel J, Kogias G, Wang AL, de Souza Silva MA, Huston JP, Zoicas I, von Hörsten S, Kornhuber J, Korth C, Müller CP. Uzuneser TC, et al. Front Psychiatry. 2019 Apr 9;10:222. doi: 10.3389/fpsyt.2019.00222. eCollection 2019. Front Psychiatry. 2019. PMID: 31057438 Free PMC article.
References
- Green MF, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56:301–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 MH094268/MH/NIMH NIH HHS/United States
- MH‑069853/MH/NIMH NIH HHS/United States
- R01 MH069853/MH/NIMH NIH HHS/United States
- MH‑085226/MH/NIMH NIH HHS/United States
- R21 MH085226/MH/NIMH NIH HHS/United States
- RC1 MH088753/MH/NIMH NIH HHS/United States
- MH‑088753/MH/NIMH NIH HHS/United States
- R01 MH092443/MH/NIMH NIH HHS/United States
- MH‑94268/MH/NIMH NIH HHS/United States
- P20 MH084018/MH/NIMH NIH HHS/United States
- MH‑084018/MH/NIMH NIH HHS/United States
- MH‑092443/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical