Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis - PubMed (original) (raw)
Imatinib-sensitive tyrosine kinases regulate mycobacterial pathogenesis and represent therapeutic targets against tuberculosis
Ruth J Napier et al. Cell Host Microbe. 2011.
Erratum in
- Cell Host Microbe. 2011 Dec 15;10(6):635
Abstract
The lengthy course of treatment with currently used antimycobacterial drugs and the resulting emergence of drug-resistant strains have intensified the need for alternative therapies against Mycobacterium tuberculosis (Mtb), the etiologic agent of tuberculosis. We show that Mtb and Mycobacterium marinum use ABL and related tyrosine kinases for entry and intracellular survival in macrophages. In mice, the ABL family tyrosine kinase inhibitor, imatinib (Gleevec), when administered prophylactically or therapeutically, reduced both the number of granulomatous lesions and bacterial load in infected organs and was also effective against a rifampicin-resistant strain. Further, when coadministered with current first-line drugs, rifampicin or rifabutin, imatinib acted synergistically. These data implicate host tyrosine kinases in entry and intracellular survival of mycobacteria and suggest that imatinib may have therapeutic efficacy against Mtb. Because imatinib targets host, it is less likely to engender resistance compared to conventional antibiotics and may decrease the development of resistance against coadministered drugs.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
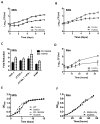
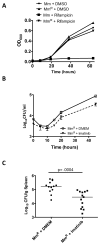
Figure 1. Src- and Abl-family TKIs reduce intracellular survival of Mtb and Mm in vitro
A,B Intracellular survival of Mtb H37Rv (MOI=10) in J774A.1 macrophage-like cells treated with PD-166326 (10 μM), an Src and Abl-family TKI, or the carrier DMSO (0.1%; control) (A) or imatinib (10μM), an Abl-family TKI (B). C Effects of PD-166326 or imatinib (each at 10 μM) on Mtb H37Rv in THP-1 cells, J774A.1 cells left untreated or treated with IFNγ, or A549 cells. Ordinate represents maximal fold reduction at 8d p.i. with respect to DMSO controls. D Intracellular survival of Mm 1218R (MOI=1) in J774A.1 cells treated with imatinib (10 μM). E,F Growth curves of Mtb H37Rv (E) or Mm 1218R (F) in 7H9 broth containing PD-166326 (10 μM), imatinib (10 μM), or DMSO (0.1%). OD600 was measured at times indicated. Data points represent the mean of three separate experiments, and error bars are +/− SEM.
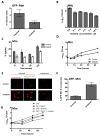
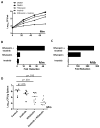
Figure 2. Abl-family TKs and other imatinib-sensitive kinases mediate intracellular survival of Mtb and Mm
A J774A.1 macrophages were infected with GFP-Mm for 2 hours in the presence or absence of imatinib (10 μM), and treated for 2 additional hours with media supplemented with amikacin to kill extracellular bacteria. The number of infected cells, measured as those containing GFP-positive bacilli, was quantified by fluorescence microscopy. B THP-1 cells were infected with Mtb and treated with imatinib at concentrations ranging from 0.1 to 10 μM for 2 h. Drug was removed, and cells were incubated for an additional 2h with amikacin to kill extracellular bacteria, and then harvested 20 hours later to determine CFU/ml. C J774A.1 macrophages were treated with imatinib (10 μM) and incubated with Mm at 4°C before returning the cells to 37°C to synchronize entry. At various times thereafter, drug was removed, and the cells treated with amikacin for 2hrs. Cells were then lysed and CFUs determined. D J774A.1 macrophages were infected with Mm 1218R (MOI=1) for 2h and then incubated with amikacin for an additional 2h. After washing, media with or without 10 μM imatinib was added, and CFU/ml determined at various time points thereafter. E,F J774A.1 cells were infected and treated as in D, stained 24 h p.i. with lysotracker red, and visualized live. The percent of GFP-Mm colocalizing with the lysotracker red labeled compartment was quantified (250 bacteria from 3 experiments). Scale bar represents 5 μM. G Fibroblasts derived from Abl1−/−/Abl2−/− mice or from age-matched wild-type mice were infected with Mm 1218R (MOI=60) for 4h. Cells were treated with amikacin for 2h, and CFU/ml determined at various time points thereafter. Imatinib or carrier was present throughout. Data represent the mean +/− SEM from 3 experiments.
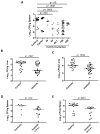
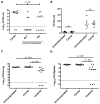
Figure 3. Imatinib reduces bacterial load in mice infected with Mm
A C57Bl/6 mice were injected in the tail vein with 105 CFU Mm 1218R. Beginning 24h prior to infection, animals were administered water (carrier) or imatinib at concentrations of 25, 50, 66.7, 100 or 200 mg/kg/day. CFU/gram was determined in spleen at d7 p.i. B,C Mice were infected with Mm as in (A) and pre-treated with carrier or with imatinib (100mg/kg/day) and CFU/g from liver (B) or lungs (C) was determined 7d p.i. D,E Mice infected with Mm as in A–C except administration of imatinib (100mg/kg/day) commenced 1 h (D) or 24 h (E) p.i. Cumulative data from at least 3 experiments are presented. Each point represents an individual mouse, and the line represents the median CFU; p values determined by a nonparametric Mann-Whitney Rank Sum test.
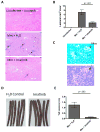
Figure 4. Imatinib reduces liver pathology and tail lesions in mice infected with Mm
A H&E-stained sections of livers of mice left uninfected and treated with 100mg/kg/day imatinib, or infected and treated with H20 or with 100mg/kg/day imatinib 1d prior to infection. Arrows depict individual lesions. Magnification x100. B Quantitation of number of lesions present in liver sections per mm2. Data were derived from 27 liver sections from mice in 3 experiments. Data are presented as mean +/− SEM. p values were determined by Mann-Whitney rank sum test. C Acid fast (upper) or H&E (lower) staining of adjacent sections of spleen taken from infected and untreated mice from Figure 3A. Arrows indicate Mm. Magnification x400. D Images of tails from mice infected i.v. with 107 CFU/ml Mm 1218R and treated with carrier (H2O) or imatinib (100mg/kg/day) for seven days beginning 24 h p.i.. Each mouse received one injection. Images are representative of 3 independent experiments. E Effects of imatinib (100mg/kg/day) on the extent of lesions on tail. Size of lesions on a particular tail were measured and summed, and the values depicted in cm. Data are represented as the mean cumulative lesion size/tail from 15 tails from each experimental group. +/− SD; p values were determined by Mann-Whitney rank sum test.
Figure 5. Imatinib reduces bacterial load in macrophages and mice infected with rifampicin-resistant Mm
A Growth curves of Mm wild-type strain (Mm) or a rifampicin-resistant Mm strain (MmR) in 7H9 media left untreated or treated with 1 μg/ml rifampicin. Cell density was measured by OD600. Data in A and B are presented as mean +/− SEM. B Intracellular survival of MmR (MOI=1) in J774A.1 macrophages in the presence or absence of imatinib (10 μM). C Effects of imatinib (100mg/kg/day) on mice infected with 105 CFU Mm strain MmR. Cumulative data from 3 independent experiments are presented. The line represents the median CFU; p values were calculated by a nonparametric Mann-Whitney Rank Sum test.
Figure 6. Imatinib and antibiotics act in synergy to reduce mycobacteria survival
A Intracellular survival of Mm (MOI=1) in J774A.1 macrophages left untreated or treated with imatinib (10 μM), or rifampicin (0.5 μg/ml), or both drugs together. CFU/ml were determined at times indicated. B Maximal fold reduction in intracellular survival of Mm in J774A.1 cells with various treatments compared to controls at d3 p.i. C Maximal fold reduction in intracellular survival of Mtb H37Rv in THP-1 cells (MOI=10) with imatinib (10 μM), rifampicin (0.125 μg/ml), or both drugs together. D Mice were administered imatinib (100mg/kg/day), or rifabutin (2.5mg/kg/day i.p.) or both drugs together. CFU in the spleen were determined at day seven p.i. Line represents the median. (n=3; data from a representative experiment are presented). The line represents the median CFU; p values were calculated by a nonparametric Mann-Whitney Rank Sum test.
Figure 7. Imatinib reduces bacterial load in mice infected with Mtb
A C57Bl/6 mice were infected with 50–100 CFU of aerosolized Mtb Erdman. Beginning 24h prior to infection, animals were administered water (carrier) or imatinib at concentrations of 66.7 mg/kg/day or 100 mg/kg/day. CFU was determined in right superior lobe of the lung at 28 days p.i. Solid lines represent the median CFU; dotted line represents the limit of detection (10 CFU). p values were determined by a nonparametric Kruskal-Wallis test. B 24h prior to infection C57Bl/6 mice were administered carrier pumps. Un-manipulated or carrier treated mice were infected with a low dose (2.5×105 CFU; left) or high dose (1×107 CF; right) of aerosolized Mtb Erdman and CFU determined in the whole lung at 24h p.i. The solid line represents the median CFU. C–D C57Bl/6 mice were infected with 2.5×105 CFU of aerosolized Mtb Erdman. Beginning 24h prior to infection, animals were left untreated, or, administered carrier (water) or imatinib at a concentration of 66.7 mg/kg/day. CFU was determined by plating homogenates of the whole lung (C) or spleen (D) at 28 days p.i. The solid line represents the median CFU; dotted line represents the limit of detection (10 CFU). p values were determined by a nonparametric Mann-Whitney test.
Similar articles
- The host-directed therapeutic imatinib mesylate accelerates immune responses to Mycobacterium marinum infection and limits pathology associated with granulomas.
Cleverley TL, Peddineni S, Guarner J, Cingolani F, Garcia PK, Koehler H, Mocarski ES, Kalman D. Cleverley TL, et al. PLoS Pathog. 2023 May 18;19(5):e1011387. doi: 10.1371/journal.ppat.1011387. eCollection 2023 May. PLoS Pathog. 2023. PMID: 37200402 Free PMC article. - Efficacy of dual-specific Bcr-Abl and Src-family kinase inhibitors in cells sensitive and resistant to imatinib mesylate.
Tipping AJ, Baluch S, Barnes DJ, Veach DR, Clarkson BM, Bornmann WG, Mahon FX, Goldman JM, Melo JV. Tipping AJ, et al. Leukemia. 2004 Aug;18(8):1352-6. doi: 10.1038/sj.leu.2403416. Leukemia. 2004. PMID: 15201856 - Abelson tyrosine kinase controls phagosomal acidification required for killing of Mycobacterium tuberculosis in human macrophages.
Bruns H, Stegelmann F, Fabri M, Döhner K, van Zandbergen G, Wagner M, Skinner M, Modlin RL, Stenger S. Bruns H, et al. J Immunol. 2012 Oct 15;189(8):4069-78. doi: 10.4049/jimmunol.1201538. Epub 2012 Sep 17. J Immunol. 2012. PMID: 22988030 Free PMC article. - Mechanisms of resistance to imatinib mesylate in Bcr-Abl-positive leukemias.
Nimmanapalli R, Bhalla K. Nimmanapalli R, et al. Curr Opin Oncol. 2002 Nov;14(6):616-20. doi: 10.1097/00001622-200211000-00005. Curr Opin Oncol. 2002. PMID: 12409651 Review. - [Development of antituberculous drugs: current status and future prospects].
Tomioka H, Namba K. Tomioka H, et al. Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
- Can host receptors for fungi be targeted for treatment of fungal infections?
Filler SG. Filler SG. Trends Microbiol. 2013 Aug;21(8):389-96. doi: 10.1016/j.tim.2013.05.006. Epub 2013 Jun 21. Trends Microbiol. 2013. PMID: 23796589 Free PMC article. Review. - All trans retinoic acid as a host-directed immunotherapy for tuberculosis.
Bahlool AZ, Grant C, Cryan SA, Keane J, O'Sullivan MP. Bahlool AZ, et al. Curr Res Immunol. 2022 Mar 30;3:54-72. doi: 10.1016/j.crimmu.2022.03.003. eCollection 2022. Curr Res Immunol. 2022. PMID: 35496824 Free PMC article. Review. - Combating Intracellular Pathogens with Repurposed Host-Targeted Drugs.
Schor S, Einav S. Schor S, et al. ACS Infect Dis. 2018 Feb 9;4(2):88-92. doi: 10.1021/acsinfecdis.7b00268. Epub 2018 Jan 3. ACS Infect Dis. 2018. PMID: 29298032 Free PMC article. - Toxoplasma gondii induces prolonged host epidermal growth factor receptor signalling to prevent parasite elimination by autophagy: Perspectives for in vivo control of the parasite.
Lopez Corcino Y, Gonzalez Ferrer S, Mantilla LE, Trikeriotis S, Yu JS, Kim S, Hansen S, Portillo JC, Subauste CS. Lopez Corcino Y, et al. Cell Microbiol. 2019 Oct;21(10):e13084. doi: 10.1111/cmi.13084. Epub 2019 Jul 17. Cell Microbiol. 2019. PMID: 31290228 Free PMC article. - Host-directed antimicrobial drugs with broad-spectrum efficacy against intracellular bacterial pathogens.
Czyż DM, Potluri LP, Jain-Gupta N, Riley SP, Martinez JJ, Steck TL, Crosson S, Shuman HA, Gabay JE. Czyż DM, et al. mBio. 2014 Jul 29;5(4):e01534-14. doi: 10.1128/mBio.01534-14. mBio. 2014. PMID: 25073644 Free PMC article.
References
- Bleed D, Dye C, Raviglione MC. Dynamics and control of the global tuberculosis epidemic. Curr Opin Pulm Med. 2000;6:174–179. - PubMed
- Bolton AE, Peng B, Hubert M, Krebs-Brown A, Capdeville R, Keller U, Seiberling M. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–106. - PubMed
- Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01AI056067-01/AI/NIAID NIH HHS/United States
- R01 DK074731/DK/NIDDK NIH HHS/United States
- R01 AI072462-03/AI/NIAID NIH HHS/United States
- CA016672/CA/NCI NIH HHS/United States
- R01 DK074731-03/DK/NIDDK NIH HHS/United States
- R01 AI056067/AI/NIAID NIH HHS/United States
- R01 AI072462-05/AI/NIAID NIH HHS/United States
- R01A107246201/PHS HHS/United States
- R01 AI072462-04/AI/NIAID NIH HHS/United States
- R01 AI072462/AI/NIAID NIH HHS/United States
- R01 AI056067-05/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous