Reductive carboxylation supports growth in tumour cells with defective mitochondria - PubMed (original) (raw)
Reductive carboxylation supports growth in tumour cells with defective mitochondria
Andrew R Mullen et al. Nature. 2011.
Abstract
Mitochondrial metabolism provides precursors to build macromolecules in growing cancer cells. In normally functioning tumour cell mitochondria, oxidative metabolism of glucose- and glutamine-derived carbon produces citrate and acetyl-coenzyme A for lipid synthesis, which is required for tumorigenesis. Yet some tumours harbour mutations in the citric acid cycle (CAC) or electron transport chain (ETC) that disable normal oxidative mitochondrial function, and it is unknown how cells from such tumours generate precursors for macromolecular synthesis. Here we show that tumour cells with defective mitochondria use glutamine-dependent reductive carboxylation rather than oxidative metabolism as the major pathway of citrate formation. This pathway uses mitochondrial and cytosolic isoforms of NADP(+)/NADPH-dependent isocitrate dehydrogenase, and subsequent metabolism of glutamine-derived citrate provides both the acetyl-coenzyme A for lipid synthesis and the four-carbon intermediates needed to produce the remaining CAC metabolites and related macromolecular precursors. This reductive, glutamine-dependent pathway is the dominant mode of metabolism in rapidly growing malignant cells containing mutations in complex I or complex III of the ETC, in patient-derived renal carcinoma cells with mutations in fumarate hydratase, and in cells with normal mitochondria subjected to acute pharmacological ETC inhibition. Our findings reveal the novel induction of a versatile glutamine-dependent pathway that reverses many of the reactions of the canonical CAC, supports tumour cell growth, and explains how cells generate pools of CAC intermediates in the face of impaired mitochondrial metabolism.
Conflict of interest statement
None of the authors have competing financial interests to declare. Reprints and permissions information is available at www.nature.com/reprints.
Figures
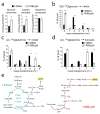
Figure 1. A reductive pathway of glutamine metabolism in cancer cells lacking activity of ETC complex III
a, Glucose utilization, lactate secretion and glutamine utilization in 143B_wt_ and 143B_cytb_ cells. b, Mass isotopomer analysis of citrate in cells cultured with D[U-13C]glucose and unlabeled glutamine. c,d, Mass isotopomer analysis of citrate and fumarate in cells cultured with L[U-13C]glutamine and unlabeled glucose. Data are the average ± S.D. for three independent cultures. * p<0.05; ** p<0.005, Student’s t-test. e, Schematic of glutamine metabolism in 143B_wt_ and 143B_cytb_ cells. Colored arrows follow the paths of glutamine-derived carbon. Abbreviations: Ac-CoA, acetyl-CoA; OAA, oxaloacetate; Gln, glutamine; Glu, glutamate; αKG, α-ketoglutarate; Succ-CoA, succinyl-CoA; Fum, fumarate; Mal, malate; PDH, pyruvate dehydrogenase; ACL, ATP-citrate lyase; IDH, isocitrate dehydrogenase; αKGDH, α-ketoglutarate dehydrogenase.
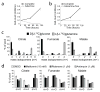
Figure 2. NADP+/NADPH-dependent isoforms of isocitrate dehydrogenase contribute to reductive carboxylation
a, Transient silencing of isocitrate dehydrogenase (IDH) proteins in 143B_cytb_ cells with short interfering RNAs (siRNAs) directed against IDH1, IDH2 or IDH3. An siRNA directed against luciferase (Luc) was used as a negative control. When IDH1 and IDH2 siRNAs were transfected concurrently (IDH1+IDH2), a transfection with the same nanomolar amount of Luc siRNA (2xLuc) was used as a negative control. b, Mass isotopomer analysis of citrate and glutamate in 143B_cytb_ cells cultured with L[U-13C]glutamine after silencing of IDH isoforms. Data are the average ± S.D. of three independent cultures. *p<0.05, Student’s t-test.
Figure 3. Glutamine is the major lipogenic precursor in cells lacking oxidative CAC function
a, Cells were cultured in medium containing 3H2O (21% volume), or 14C tracers of acetate (ac, 5 μCi), glucose (glc, 10 μCi) or glutamine (gln, 10 μCi). Lipids were analyzed for 3H or 14C content by scintillation counting. In the 14C experiments, raw counts per thousand cells were normalized to the absolute rate of fatty acid synthesis established using 3H2O. *p<0.05, Student’s t-test. b, Schematic outlining synthesis of fatty acids (FA) from acetyl-CoA (Ac-CoA) and the source of the triplet and doublet at ω-1 in 13C NMR spectroscopy. c,d, 13C NMR of lipids labeled with D[U-13C]glucose (c) or L[U-13C]glutamine (d). Insets are expansions of the ω-1 resonance. Abbreviations: s, singlet; d, doublet; t, triplet.
Figure 4. Glutamine-dependent reductive carboxylation in renal carcinoma cells lacking fumarate hydratase and during ETC inhibition in tumor cells with normal mitochondria
Growth of UOK262 renal carcinoma cells in complete medium, in medium in which glucose was replaced with galactose (a), and in medium lacking glutamine (b). c, Mass isotopomer analysis of citrate, fumarate and malate from UOK262 cells cultured in medium containing D[U-13C]glucose and unlabeled glutamine, or L[U-13C]glutamine and unlabeled glucose. d, Mass isotopomer analysis for citrate, fumarate and malate from 143B_wt_ cells cultured in medium containing unlabeled glucose and L[U-13C]glutamine. The labeled glutamine was introduced at the same time the cells were treated with vehicle (DMSO), metformin, rotenone or antimycin. Metabolites were extracted 6 hours later. In all experiments, data are the average ± S.D. of three independent cultures.
Similar articles
- Reductive carboxylation supports redox homeostasis during anchorage-independent growth.
Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, Schmidt S, Metallo CM, Dranka BP, Schwartz B, DeBerardinis RJ. Jiang L, et al. Nature. 2016 Apr 14;532(7598):255-8. doi: 10.1038/nature17393. Epub 2016 Apr 6. Nature. 2016. PMID: 27049945 Free PMC article. - Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia.
Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Metallo CM, et al. Nature. 2011 Nov 20;481(7381):380-4. doi: 10.1038/nature10602. Nature. 2011. PMID: 22101433 Free PMC article. - Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability.
Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Wise DR, et al. Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19611-6. doi: 10.1073/pnas.1117773108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106302 Free PMC article. - Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer.
Corbet C, Feron O. Corbet C, et al. Curr Opin Clin Nutr Metab Care. 2015 Jul;18(4):346-53. doi: 10.1097/MCO.0000000000000178. Curr Opin Clin Nutr Metab Care. 2015. PMID: 26001655 Review. - Mitochondrial dysfunctions in cancer: genetic defects and oncogenic signaling impinging on TCA cycle activity.
Desideri E, Vegliante R, Ciriolo MR. Desideri E, et al. Cancer Lett. 2015 Jan 28;356(2 Pt A):217-23. doi: 10.1016/j.canlet.2014.02.023. Epub 2014 Mar 12. Cancer Lett. 2015. PMID: 24614286 Review.
Cited by
- The emerging role of fumarate as an oncometabolite.
Yang M, Soga T, Pollard PJ, Adam J. Yang M, et al. Front Oncol. 2012 Jul 31;2:85. doi: 10.3389/fonc.2012.00085. eCollection 2012. Front Oncol. 2012. PMID: 22866264 Free PMC article. - Spatial single-cell isotope tracing reveals heterogeneity of de novo fatty acid synthesis in cancer.
Buglakova E, Ekelöf M, Schwaiger-Haber M, Schlicker L, Molenaar MR, Shahraz M, Stuart L, Eisenbarth A, Hilsenstein V, Patti GJ, Schulze A, Snaebjornsson MT, Alexandrov T. Buglakova E, et al. Nat Metab. 2024 Sep;6(9):1695-1711. doi: 10.1038/s42255-024-01118-4. Epub 2024 Sep 9. Nat Metab. 2024. PMID: 39251875 Free PMC article. - TORquing metabolic reprogramming in cancer cells.
Pusapati R, Settleman J. Pusapati R, et al. Cell Cycle. 2016 Sep 16;15(18):2387-8. doi: 10.1080/15384101.2016.1204850. Epub 2016 Jun 30. Cell Cycle. 2016. PMID: 27362787 Free PMC article. No abstract available. - Identification of purine biosynthesis as an NADH-sensing pathway to mediate energy stress.
Yang R, Yang C, Ma L, Zhao Y, Guo Z, Niu J, Chu Q, Ma Y, Li B. Yang R, et al. Nat Commun. 2022 Nov 17;13(1):7031. doi: 10.1038/s41467-022-34850-0. Nat Commun. 2022. PMID: 36396642 Free PMC article. - Metabolic plasticity maintains proliferation in pyruvate dehydrogenase deficient cells.
Rajagopalan KN, Egnatchik RA, Calvaruso MA, Wasti AT, Padanad MS, Boroughs LK, Ko B, Hensley CT, Acar M, Hu Z, Jiang L, Pascual JM, Scaglioni PP, DeBerardinis RJ. Rajagopalan KN, et al. Cancer Metab. 2015 Jun 29;3:7. doi: 10.1186/s40170-015-0134-4. eCollection 2015. Cancer Metab. 2015. PMID: 26137220 Free PMC article.
References
- Baysal BE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK072565/DK/NIDDK NIH HHS/United States
- T32 CA009560/CA/NCI NIH HHS/United States
- T32CA009560/CA/NCI NIH HHS/United States
- DK078933/DK/NIDDK NIH HHS/United States
- T32 GM008061/GM/NIGMS NIH HHS/United States
- T32 GM083831/GM/NIGMS NIH HHS/United States
- R01 CA123067/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- RR02584/RR/NCRR NIH HHS/United States
- R01CA123067/CA/NCI NIH HHS/United States
- K01 DK078933/DK/NIDDK NIH HHS/United States
- K08DK072565/DK/NIDDK NIH HHS/United States
- 5T32GM083831/GM/NIGMS NIH HHS/United States
- P41 RR002584/RR/NCRR NIH HHS/United States
- R01 CA157996/CA/NCI NIH HHS/United States
- R01CA157996/CA/NCI NIH HHS/United States
- T32GM008061/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous