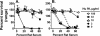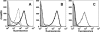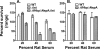Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis - PubMed (original) (raw)
Enhanced bacteremia in human factor H transgenic rats infected by Neisseria meningitidis
David M Vu et al. Infect Immun. 2012 Feb.
Abstract
Neisseria meningitidis binds the complement downregulating protein, factor H (fH), which enables the organism to evade host defenses. Two fH ligands, fHbp and NspA, are known to bind specifically to human fH. We developed a human fH transgenic infant rat model to investigate the effect of human fH on meningococcal bacteremia. At 18 h after intraperitoneal challenge with 560 CFU of group B strain H44/76, all 19 human fH-positive rats had positive blood cultures compared to 0 of 7 human fH-negative control littermates (P < 0.0001). Human fH-positive infant rats also developed bacteremia after challenge with isogenic mutants of H44/76 in which genes encoding fHbp and NspA (ΔfHbp ΔNspA mutant) or the lipooligosaccharide sialyltransferase (Δlst mutant) had been inactivated. A fully encapsulated ΔfHbp ΔNspA Δlst mutant unable to sialylate lipooligosaccharide or bind human fH via the known fH ligands did not cause bacteremia, which argued against global susceptibility to bacteremia resulting from random integration of the transgene into the rat genome. In vitro, the wild-type and ΔfHbp ΔNspA mutant strains were killed by as little as 20% wild-type infant rat serum. The addition of 3 μg of human fH/ml permitted survival of the wild-type strain in up to 60% infant rat serum, whereas ≥33 μg of human fH/ml was required to rescue the ΔfHbp ΔNspA mutant. The ability of meningococci lacking expression of fHbp and NspA to cause invasive disease in human fH transgenic rats and to survive in wild-type infant rat serum supplemented with human fH indicates an additional human fH-dependent mechanism of evasion of innate immunity.
Figures
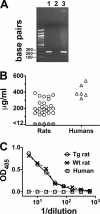
Fig 1
Construction of human fH transgenic rat line. (A) PCR confirmation of the presence of the human fH cDNA in genomic DNA isolated from rat tails. Lane 1, amplification product (232 bp) from genomic DNA extracted from a human factor H transgenic rat; lane 2, amplification product from genomic DNA extracted from a wild-type rat; and lane 3, amplification product from a plasmid containing human factor H cDNA. (B) Serum human factor H concentrations measured in infant rats, aged 3 to 4 days by a capture ELISA. Open circles, human fH-positive rats; gray-filled circles, human fH-negative rats (<12 μg of human fH/ml); open triangles, fH concentrations in control sera from healthy adult humans. The mean human fH concentration in the positive rat sera was lower than that in sera of healthy human adults (P < 0.0001). (C) Serum rat fH measured using a capture ELISA specific for rodent fH. Open circles, solid line, pooled sera from three human fH-positive transgenic rats. Crosses with dashed line, pooled sera from three wild-type rats. Open boxes, dotted line, pooled sera from three human adults.
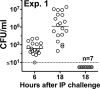
Fig 2
Meningococcal bacteremia in human fH transgenic rats (experiment 1). Six- to seven-day-old animals were challenged i.p. with 560 CFU of group B strain H44/76. Blood cultures were obtained from the human-fH positive rats at 6 or 18 h after challenge (individual animals were bled once). For human fH-negative animals, cultures were obtained only at 18 h because of insufficient numbers of animals. Open circles, CFU/ml of human fH-positive animals; gray-filled circles, CFU/ml of human fH-negative animals. Dotted line indicates the lower limit of detection for positive blood cultures (10 CFU/ml). At 6 h, the geometric mean (horizontal solid line) of the CFU/ml of blood was 394, which increased to 1.2 × 105 CFU/ml at 18 h (P < 0.0001). At 18 h, none of the human fH-negative animals had positive cultures of blood (0 of 7 versus 19 of 19 human fH-positive animals at 18 h, P < 0.0001 [Fisher exact test]).
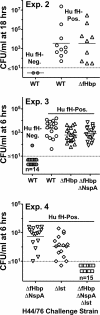
Fig 3
Meningococcal bacteremia in human fH transgenic rats after i.p. challenge of infant rats (experiments 2, 3, and 4). Rats were challenged with a wild-type (WT) strain of H44/76 or isogenic knockout mutants. Blood cultures were obtained at 18 h (experiment 2) or 6 h after the challenges (experiments 3 and 4). The ages of rats at the time of challenge in each experiment and the CFU/rat used for the challenges are summarized in Table 2. Gray-filled circles, CFU/ml of individual human fH-negative rats (experiments 2 and 3 only); open symbols, CFU/ml of individual human fH-positive rats; horizontal solid lines, geometric means of the CFU/ml of the respective groups; horizontal dotted line, limit of detection of bacteremia. For a more detailed description of the statistical analyses of each experiment, see Table 2.
Fig 4
Effect of the addition of human fH on survival of H44/76 bacteria after 60 min of incubation with pooled sera from 8- to 9-day-old wild-type rats. (A) Wild-type strain; (B), ΔfHbp ΔNspA mutant. Exogenous human fH concentrations: X's with solid line, 0 μg/ml; closed circles with dotted line, 3 μg/ml; open triangles with dashed line, 10 μg/ml; gray-filled boxes with solid line, 33 μg/ml; open circles with dashed line, 100 μg/ml. The data points represent median values from triplicate measurements. Error bars represent the range from three replicate measurements (in cases where two of the three respective results were identical, the bars extend in only one direction, above or below the median value). Similar results were obtained in a separate independent experiment (not shown).
Fig 5
Binding of human fH on the surfaces of live meningococci as measured by flow cytometry. (A and B) Binding of control MAbs. (A) H44/76 wild-type strain. Bacteria alone, gray-shaded area; anti-porin P1.7 MAb, thick black line; anti-fHbp MAb JAR 3, dashed black line; anti-NspA MAb 14C7, dark gray line. (B) H44/76 ΔfHbp ΔNspA double-knockout mutant. Symbols are as defined for panel A. (C) Binding of human fH to live bacteria. Wild-type H44/76 strain with 60% infant rat serum and 100 μg of human fH/ml, dark gray line; ΔfHbp ΔNspA double-knockout mutant with 60% infant rat serum and 100 μg of human fH/ml, black line; wild-type or ΔfHbp ΔNspA double-knockout mutant without human fH and without infant rat serum; dashed line and shaded gray areas, respectively.
Fig 6
Effect of sialylated LOS on survival of N. meningitidis in wild-type infant rat serum supplemented with human fH. Rat serum pool was from 8- to 9-day-old animals. Human fH was added to a final concentration of 100 μg/ml to each dilution of rat serum. (A) Open bars, H44/76 wild-type strain; Gray bars, Δ_lst_ isogenic mutant that lacks a gene required for terminal sialylation of LOS; diagonal hatched bars, ΔfHbp ΔNspA Δ_lst_ triple-knockout mutant that, in addition to not binding fH to fHbp or NspA, is unable to sialylate LOS. (B) Open bars, H44/76 wild-type strain; horizontal hatched bars, ΔfHbp ΔNspA double mutant. The data in panels A and B are from separate experiments, each replicated two or three times. In panels A and B, bars represent median values from triplicate measurements in an individual experiment. Error bars represent ranges.
Similar articles
- fH-dependent complement evasion by disease-causing meningococcal strains with absent fHbp genes or frameshift mutations.
Giuntini S, Vu DM, Granoff DM. Giuntini S, et al. Vaccine. 2013 Aug 28;31(38):4192-9. doi: 10.1016/j.vaccine.2013.06.009. Epub 2013 Jun 17. Vaccine. 2013. PMID: 23791680 Free PMC article. - The meningococcal vaccine candidate neisserial surface protein A (NspA) binds to factor H and enhances meningococcal resistance to complement.
Lewis LA, Ngampasutadol J, Wallace R, Reid JE, Vogel U, Ram S. Lewis LA, et al. PLoS Pathog. 2010 Jul 29;6(7):e1001027. doi: 10.1371/journal.ppat.1001027. PLoS Pathog. 2010. PMID: 20686663 Free PMC article. - Factor H-dependent alternative pathway inhibition mediated by porin B contributes to virulence of Neisseria meningitidis.
Lewis LA, Vu DM, Vasudhev S, Shaughnessy J, Granoff DM, Ram S. Lewis LA, et al. mBio. 2013 Oct 15;4(5):e00339-13. doi: 10.1128/mBio.00339-13. mBio. 2013. PMID: 24129254 Free PMC article. - Does binding of complement factor H to the meningococcal vaccine antigen, factor H binding protein, decrease protective serum antibody responses?
Granoff DM, Ram S, Beernink PT. Granoff DM, et al. Clin Vaccine Immunol. 2013 Aug;20(8):1099-107. doi: 10.1128/CVI.00260-13. Epub 2013 Jun 5. Clin Vaccine Immunol. 2013. PMID: 23740919 Free PMC article. Review. - Meningococcal factor H binding protein as immune evasion factor and vaccine antigen.
Principato S, Pizza M, Rappuoli R. Principato S, et al. FEBS Lett. 2020 Aug;594(16):2657-2669. doi: 10.1002/1873-3468.13793. Epub 2020 May 12. FEBS Lett. 2020. PMID: 32298465 Review.
Cited by
- Risk of Invasive Meningococcal Disease in Men Who Have Sex with Men: Lessons Learned from an Outbreak in Germany, 2012-2013.
Hellenbrand W, Claus H, Schink S, Marcus U, Wichmann O, Vogel U. Hellenbrand W, et al. PLoS One. 2016 Aug 3;11(8):e0160126. doi: 10.1371/journal.pone.0160126. eCollection 2016. PLoS One. 2016. PMID: 27486669 Free PMC article. - Effect of complement Factor H on antibody repertoire and protection elicited by meningococcal capsular group B vaccines containing Factor H binding protein.
Beernink PT. Beernink PT. Hum Vaccin Immunother. 2020 Mar 3;16(3):703-712. doi: 10.1080/21645515.2019.1664241. Epub 2019 Oct 22. Hum Vaccin Immunother. 2020. PMID: 31526219 Free PMC article. - Heterogeneity in rhesus macaque complement factor H binding to meningococcal factor H binding protein (FHbp) informs selection of primates to assess immunogenicity of FHbp-based vaccines.
Beernink PT, Shaughnessy J, Stefek H, Ram S, Granoff DM. Beernink PT, et al. Clin Vaccine Immunol. 2014 Nov;21(11):1505-11. doi: 10.1128/CVI.00517-14. Epub 2014 Sep 3. Clin Vaccine Immunol. 2014. PMID: 25185576 Free PMC article. - Lipooligosaccharide Structures of Invasive and Carrier Isolates of Neisseria meningitidis Are Correlated with Pathogenicity and Carriage.
John CM, Phillips NJ, Din R, Liu M, Rosenqvist E, Høiby EA, Stein DC, Jarvis GA. John CM, et al. J Biol Chem. 2016 Feb 12;291(7):3224-38. doi: 10.1074/jbc.M115.666214. Epub 2015 Dec 11. J Biol Chem. 2016. PMID: 26655715 Free PMC article.
References
- Agarwal S, et al. 2011. Linkage specificity and role of properdin in activation of the alternative complement pathway by fungal glycans. mBio 2 (5): e00178–111 doi:10.01128/mBio.00178–111. - DOI - PMC - PubMed
- Borrow R, et al. 2005. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin. Diagn. Lab. Immunol. 12:970–976 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI 082263/AI/NIAID NIH HHS/United States
- AI 084048/AI/NIAID NIH HHS/United States
- R37 AI032725/AI/NIAID NIH HHS/United States
- C06 RR 016226/RR/NCRR NIH HHS/United States
- R56 AI032725/AI/NIAID NIH HHS/United States
- R01 AI046464/AI/NIAID NIH HHS/United States
- AI 032725/AI/NIAID NIH HHS/United States
- R01 AI082263/AI/NIAID NIH HHS/United States
- C06 RR016226/RR/NCRR NIH HHS/United States
- R01 AI032725/AI/NIAID NIH HHS/United States
- R01 AI 046464/AI/NIAID NIH HHS/United States
- R01 AI054544/AI/NIAID NIH HHS/United States
- L40 AI057605/AI/NIAID NIH HHS/United States
- AI 054544/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous