Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes - PubMed (original) (raw)
. 2011 Dec;121(12):4796-809.
doi: 10.1172/JCI60405. Epub 2011 Nov 21.
Affiliations
- PMID: 22105175
- PMCID: PMC3223072
- DOI: 10.1172/JCI60405
Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes
Yoshiyuki Kawashima et al. J Clin Invest. 2011 Dec.
Abstract
Inner ear hair cells convert the mechanical stimuli of sound, gravity, and head movement into electrical signals. This mechanotransduction process is initiated by opening of cation channels near the tips of hair cell stereocilia. Since the identity of these ion channels is unknown, and mutations in the gene encoding transmembrane channel-like 1 (TMC1) cause hearing loss without vestibular dysfunction in both mice and humans, we investigated the contribution of Tmc1 and the closely related Tmc2 to mechanotransduction in mice. We found that Tmc1 and Tmc2 were expressed in mouse vestibular and cochlear hair cells and that GFP-tagged TMC proteins localized near stereocilia tips. Tmc2 expression was transient in early postnatal mouse cochlear hair cells but persisted in vestibular hair cells. While mice with a targeted deletion of Tmc1 (Tmc1(Δ) mice) were deaf and those with a deletion of Tmc2 (Tmc2(Δ) mice) were phenotypically normal, Tmc1(Δ)Tmc2(Δ) mice had profound vestibular dysfunction, deafness, and structurally normal hair cells that lacked all mechanotransduction activity. Expression of either exogenous TMC1 or TMC2 rescued mechanotransduction in Tmc1(Δ)Tmc2(Δ) mutant hair cells. Our results indicate that TMC1 and TMC2 are necessary for hair cell mechanotransduction and may be integral components of the mechanotransduction complex. Our data also suggest that persistent TMC2 expression in vestibular hair cells may preserve vestibular function in humans with hearing loss caused by TMC1 mutations.
Figures
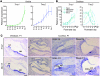
Figure 1. Hair cell expression of Tmc1 and Tmc2.
(A) qPCR analysis of Tmc1 and Tmc2 in RNA extracted from mouse utricles at developmental ages between E15 and P21. We used primers that amplify a fragment of Tmc1 common to both Tmc1ex1 and Tmc1ex2. Each sample was analyzed 6–9 times. Expression levels were normalized, using the ΔΔCt method, first to Actb (encoding β-actin) expression and then to the Tmc expression level at E15. Error bars indicate ± SD. (B) qPCR analysis of mouse cochlear RNA showed a steady rise in Tmc1 expression through P5 and in the P22 apical sample. Other portions of P22 cochleae were not harvested due to rapid degradation of hair cell integrity and mRNA quality. Tmc2 RNA was transiently expressed in mouse cochleae during the postnatal period. Error bars indicate SD. (C) In situ hybridization analysis of Tmc2 in P1 mouse vestibule and cochlea. Tmc2 expression was detected in wild-type crista ampullaris (CA), utricular macula (UM), hair cells of the basal cochlear turn, and, to a lesser extent, in the middle turn at P1. Tmc2 expression was not detected in hair cells of the apical turn. Adjacent sections probed for Myo15 transcripts are shown as a control for hair cell hybridization (52). Scale bar: 100 μm. See also Supplemental Figure 1.
Figure 2. _Tmc1_Δ and _Tmc2_Δ mice.
(A) Generation of _Tmc1_Δ mice. Schematic diagram of the Tmc1 genomic locus, targeting vector, and targeted locus before and after Cre-mediated excision of PGK-Neo. 5′ and 3′ hybridization probes and restriction digestion sites for Southern blot analysis are indicated. TK encodes thymidine kinase. (B) Generation of _Tmc2_Δ mice. (C) Southern blot analyses of Tmc1 targeting. Genomic DNA was digested with NcoI for 5′ probe hybridization and EcoRV for 3′ probe hybridization. (D) Southern blot analyses of Tmc2 targeting. Genomic DNA was digested with NheI and KpnI for 5′ probe hybridization and BglI and HindIII for 3′ probe hybridization. The sizes of the _Tmc1_Δ
and _Tmc2_Δ hybridizing bands confirmed proper integration of the targeting constructs. See also Supplemental Figure 2.
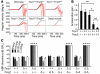
Figure 3. _Tmc1_Δ and _Tmc2_Δ hearing and balance phenotype.
(A) Representative VOR traces. Black traces show head angular velocity (degrees per second [deg/s]) during passive, whole-body rotations delivered in darkness about an Earth-vertical axis with the animal’s horizontal semicircular canals in an Earth-horizontal plane. The red traces show angular velocity of the eye during more than 16 VOR-mediated counter-rotations. Head angular velocity is inverted to facilitate visual comparison. Reflexive eye movements during the first 100 ms of the head rotation stimulus are a sensitive measure of semicircular canal (crista ampullaris) function. _Tmc1+/+Tmc2_Δ/Δ and _Tmc1_Δ/+_Tmc2_Δ/Δ mice had reduced responses compared with those of a wild-type mouse, whereas the _Tmc1_Δ/Δ_Tmc2_Δ/Δ
mouse and a dead mouse control had no response. (B) Mean (±SD) acceleration gains in VOR (*P < 0.05, **P < 0.01, Student’s t test). (C) Mean (±SD) ABR thresholds for tone-burst stimuli of 8 kHz, 16 kHz, or 32 kHz, or click stimuli in better-hearing ears of 12-week-old mice. Mice lacking functional Tmc1 had profound hearing loss, with no effect of Tmc2 genotype. *P < 0.001, 1-way ANOVA, compared with wild-type values. See also Supplemental Figure 3.
Figure 4. Hair bundle phenotype of _Tmc1_Δ/Δ and _Tmc2_Δ/Δ mice.
Vestibular and auditory hair bundle and tip-link phenotype of P3 wild-type and _Tmc1_Δ/Δ_Tmc2_Δ/Δ mice. We examined hair bundles of 20 cochleae, 10 utricles, 10 saccules, and 30 cristae ampullaris harvested from _Tmc1_Δ/Δ_Tmc2_Δ/Δ mice at P3. _Tmc1_Δ/Δ_Tmc2_Δ/Δ mice had hair bundles and tip links that were indistinguishable from those of wild-type mice. Tenting of tips of shorter stereocilia is a normal indication of tension exerted by tip links. SM, saccular macula; UM, utricular macula; CA, crista ampullaris. Arrowheads indicate tip links. Scale bars: 10 μm (top row); 250 nm (second and fourth rows); 1 μm (third row). See also Supplemental Figure 4.
Figure 5. _Tmc1_Δ and _Tmc2_Δ vestibular hair cell mechanotransduction currents.
(A) Representative mechanotransduction current families for utricle type II hair cells harvested between P1 and P8. Hair bundles were deflected in 250-ms steps that ranged from –0.5 μm to 1 μm in 0.1-μm increments. The envelope of the stimulus protocol is shown at the bottom left. Currents were recorded at –64 mV, a physiologically relevant holding potential. Some traces have been removed for clarity. Scale bars apply to all current families. (B) Summary bar graph from 111 type II utricle hair cells. The graph shows mean maximal mechanotransduction current amplitudes (±SEM). For each bar, the upper number indicates the number of mice examined, and the lower number indicates the total number hair cells examined for each genotype. *P < 0.001, 1-way ANOVA), relative to wild-type values.
Figure 6. _Tmc1_Δ and _Tmc2_Δ cochlear hair cell mechanotransduction currents.
(A) Representative mechanotransduction current families recorded from OHCs harvested at P5–P7 from the apical cochlea of mice of the indicated Tmc1;Tmc2 genotypes. Hair bundles were deflected for 90-ms steps that ranged from 200 nm to 750 nm in 50-nm increments while cells were held at a physiologically relevant holding potential (–64 mV). Some traces have been removed for clarity. The envelope of the stimulus protocol is shown at the bottom right. Tmc1_Δ/Δ_Tmc2_Δ/Δ OHCs lack mechanotransduction currents entirely. Scale bars apply to all current families. (B) Summary bar graph of mean maximal mechanotransduction current amplitudes (±SEM) from 100 apical OHCs. For each bar, the upper number indicates the number of mice examined, and the lower number indicates the total number of hair cells examined. Tmc1_Δ/Δ_Tmc2+/+ and Tmc1_Δ/Δ_Tmc2_Δ/+ hair cells had intermediate current amplitudes. *P < 0.001, 1-way ANOVA, relative to wild-type values. (C) Representative transduction currents recorded from OHCs excised at P2–P4 from the basal end of the cochlea from mice of the indicated genotypes. The stimulus protocol and scale bars in A apply. (D) Summary bar graph of maximal mechanotransduction current amplitudes (±SEM) from 45 basal OHCs. Upper numbers indicate the numbers of mice examined, and lower numbers indicate the total numbers of cells examined. *P < 0.001, 1-way ANOVA, relative to wild-type values. See also Supplemental Figure 5.
Figure 7. Rescue of mechanotransduction with Tmc1 or Tmc2.
(A) Organotypic cultures of utricles harvested at P0–P3 from _Tmc1_Δ/Δ_Tmc2_Δ/Δ mice were exposed to adenoviral vectors expressing Tmc1ex1 (n = 4 mice), Tmc1ex2 (n = 3 mice), Tmc2 (n = 5 mice), or Tmc1ex2-dn (n = 3 mice) and maintained in culture for 2–4 days. Scale bars apply to all current families. The envelope of the stimulus protocol is shown at the bottom left. (B) For each bar, the upper number indicates the number of cells with measurable mechanotransduction currents, while the lower number indicates total number of tested cells. Mean maximal currents are plotted from cells with measurable current (±SEM). (C) Organotypic cultures of cochleae were harvested from _Tmc1_Δ/Δ_Tmc2_Δ/Δ mice at P0. Representative mechanotransduction current families for cochlear OHCs transfected with CMV promoter–driven Ad-Tmc1ex1 (n = 4 mice) or MYO7A promoter–driven Ad-Tmc2 (n = 11 mice). Ad-Tmc1ex1 or Ad-Tmc2 rescue mechanotransduction in _Tmc1_Δ/Δ_Tmc2_Δ/Δ OHCs. Scale bars apply to both current families. The envelope of the stimulus protocol is shown at the bottom left. (D) Summary bar graph shows mean maximal mechanotransduction current amplitudes (±SEM) from 43 RFP-positive (transfected) OHCs. For each bar, the upper number indicates the number of cells with measurable mechanotransduction currents, while the lower number indicates total number of cells examined.
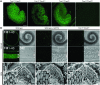
Figure 8. _Tmc1_Δ and _Tmc2_Δ hair cell uptake of FM1-43.
(A) Confocal microscopic images of P3 mouse utricles (Tmc1_Δ/+Tmc2_Δ_/+, n = 4 utricles; Tmc1_Δ/+Tmc2_Δ/Δ, n = 6; Tmc1_Δ/Δ_Tmc2_Δ/+, n = 4; Tmc1_Δ/Δ_Tmc2_Δ/_Δ, n = 6) exposed to 5 μM FM1-43. Scale bar: 100 μm. (B) IHCs and OHCs of P3 wild-type mice (n = 5 mice) had robust uptake of FM1-43. Wild-type hair cells (n = 5 mice) pretreated with 5 mM BAPTA did not take up FM1-43, and tip links are missing (tip links indicated by white arrowheads in insets). No FM1-43 uptake was detected in Tmc1_Δ/Δ_Tmc2_Δ/_Δ hair cells (n = 5 mice) despite intact hair bundles and tip links (compare insets). Black arrowheads, IHCs (middle turn); white arrowheads, OHCs (middle turn); DIC, differential interference contrast. Gamma settings for green channel were adjusted equally throughout entire images and for all images using Adobe Photoshop CS5 software. Scale bars: 100 μm, upper panels; 10 μm, middle panels; 1 μm, lower panels; 500 nm, insets in bottom row of panels. See also Supplemental Figure 6.
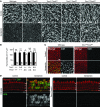
Figure 9. _Tmc1_Δ and _Tmc2_Δ hair cell uptake of gentamicin.
(A) After a 48-hour incubation in culture medium with 1 mM gentamicin, wild-type utricular hair bundles were almost completely eliminated, while Tmc1_Δ/Δ_Tmc2_Δ/Δ utricular hair cells retained an intact hair bundle. Scale bar: 10 μm. (B) The mean density of intact hair bundles (±SD) within the central area of more than 6 utricular maculae of each genotype is shown for each genotype. Tmc1_Δ/+Tmc2_Δ/_Δ and Tmc1_Δ/Δ_Tmc2_Δ/_Δ utricular maculae showed no significant difference between cultures grown with gentamicin in comparison with control cultures grown without gentamicin. Tmc1+/+Tmc2_Δ/_Δ utricular hair cells showed intermediate sensitivity to gentamicin. One-way ANOVA results for differences from normal control values are indicated: *P < 0.05; ***_P_ < 0.001; NS, _P_ > 0.05. (C) After a 6-hour incubation in culture medium with 1 mM gentamicin, robust immunoreactivity for gentamicin was detected in wild-type utricular hair cells, whereas no immunoreactivity for gentamicin was detected in Tmc1_Δ/Δ_Tmc2_Δ/_Δ hair cells. Red, anti–myosin VI antibodies; green, anti-gentamicin antibodies. Scale bar: 10 μm. (D) After a 6-hour incubation in 1 mM gentamicin, robust immunoreactivity for gentamicin was detected in wild-type IHCs and OHCs, whereas no immunoreactivity for gentamicin was detected in Tmc1_Δ/Δ_Tmc2_Δ/_Δ
hair cells. Red, anti–myosin VI antibodies; green, anti-gentamicin antibodies. Filled arrowheads, IHCs; open arrowheads, OHCs. Scale bar: 10 μm. See also Supplemental Figures 7 and 8.
Figure 10. Localization of TMC2::AcGFP.
(A) Rat utricular hair cell transfected with MYO7A-Tmc2::AcGFP at P3 plus 1 day in vitro (P3+1DIV) and incubated for 48 hours. TMC2::AcGFP accumulates toward the tips of stereocilia. Scale bar: 5 μm. Gamma settings for red and green channels were adjusted equally throughout entire images using Adobe Photoshop CS5. (B) Rat saccular hair cell transfected with MYO7A-Tmc2::AcGFP at P3+1DIV and incubated for 48 hours. TMC2::AcGFP accumulates toward the tips of stereocilia. Scale bar: 5 μm. (C) Tmc1_Δ/Δ_Tmc2_Δ/_Δ
inner hair cells transfected with MYO7A-Tmc2::AcGFP at P0+1DIV and incubated for 72 hours. TMC2::AcGFP expression was detected on stereocilia. Gamma settings for red and green channels were adjusted equally throughout the entire image using Adobe Photoshop CS5. Scale bar: 5 μm. (D) Rat OHCs transfected with MYO7A-Tmc2::AcGFP at P0+1DIV and incubated for 72 hours. TMC2::AcGFP accumulates toward the tips of stereocilia. Scale bar: 5 μm. Gamma settings for red and green channels were adjusted equally throughout the entire image using Adobe Photoshop CS5. Cumulative totals of approximately 80 of 100 TMC2::AcGFP-positive hair cells each from cochleae, saccules, and utricles displayed green fluorescence at stereocilia tips. See also Supplemental Figure 9.
Comment in
- Perception of sound and gravity by TMC1 and TMC2.
Lin X. Lin X. J Clin Invest. 2011 Dec;121(12):4633-6. doi: 10.1172/JCI61167. Epub 2011 Nov 21. J Clin Invest. 2011. PMID: 22105165 Free PMC article.
Similar articles
- Perception of sound and gravity by TMC1 and TMC2.
Lin X. Lin X. J Clin Invest. 2011 Dec;121(12):4633-6. doi: 10.1172/JCI61167. Epub 2011 Nov 21. J Clin Invest. 2011. PMID: 22105165 Free PMC article. - TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia.
Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, Millis BA, Cui R, Nakanishi H, Fujikawa T, Kawashima Y, Choi BY, Monahan K, Holt JR, Griffith AJ, Kachar B. Kurima K, et al. Cell Rep. 2015 Sep 8;12(10):1606-17. doi: 10.1016/j.celrep.2015.07.058. Epub 2015 Aug 28. Cell Rep. 2015. PMID: 26321635 Free PMC article. - Tmc2 expression partially restores auditory function in a mouse model of DFNB7/B11 deafness caused by loss of Tmc1 function.
Nakanishi H, Kurima K, Pan B, Wangemann P, Fitzgerald TS, Géléoc GS, Holt JR, Griffith AJ. Nakanishi H, et al. Sci Rep. 2018 Aug 14;8(1):12125. doi: 10.1038/s41598-018-29709-8. Sci Rep. 2018. PMID: 30108230 Free PMC article. - Transmembrane channel-like (TMC) genes are required for auditory and vestibular mechanosensation.
Kawashima Y, Kurima K, Pan B, Griffith AJ, Holt JR. Kawashima Y, et al. Pflugers Arch. 2015 Jan;467(1):85-94. doi: 10.1007/s00424-014-1582-3. Epub 2014 Jul 31. Pflugers Arch. 2015. PMID: 25074487 Free PMC article. Review. - Is TMC1 the Hair Cell Mechanotransducer Channel?
Fettiplace R. Fettiplace R. Biophys J. 2016 Jul 12;111(1):3-9. doi: 10.1016/j.bpj.2016.05.032. Biophys J. 2016. PMID: 27410728 Free PMC article. Review.
Cited by
- Molecular specializations underlying phenotypic differences in inner ear hair cells of zebrafish and mice.
Giffen KP, Liu H, Yamane KL, Li Y, Chen L, Kramer KL, Zallocchi M, He DZ. Giffen KP, et al. Front Neurol. 2024 Oct 17;15:1437558. doi: 10.3389/fneur.2024.1437558. eCollection 2024. Front Neurol. 2024. PMID: 39484049 Free PMC article. - Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae.
Tsubouchi A, Caldwell JC, Tracey WD. Tsubouchi A, et al. Curr Biol. 2012 Nov 20;22(22):2124-34. doi: 10.1016/j.cub.2012.09.019. Epub 2012 Oct 25. Curr Biol. 2012. PMID: 23103192 Free PMC article. - Designer aminoglycosides prevent cochlear hair cell loss and hearing loss.
Huth ME, Han KH, Sotoudeh K, Hsieh YJ, Effertz T, Vu AA, Verhoeven S, Hsieh MH, Greenhouse R, Cheng AG, Ricci AJ. Huth ME, et al. J Clin Invest. 2015 Feb;125(2):583-92. doi: 10.1172/JCI77424. Epub 2015 Jan 2. J Clin Invest. 2015. PMID: 25555219 Free PMC article. - HCN1 and HCN2 proteins are expressed in cochlear hair cells: HCN1 can form a ternary complex with protocadherin 15 CD3 and F-actin-binding filamin A or can interact with HCN2.
Ramakrishnan NA, Drescher MJ, Khan KM, Hatfield JS, Drescher DG. Ramakrishnan NA, et al. J Biol Chem. 2012 Nov 2;287(45):37628-46. doi: 10.1074/jbc.M112.375832. Epub 2012 Sep 4. J Biol Chem. 2012. PMID: 22948144 Free PMC article. - Hearing loss in mice with disruption of auditory epithelial patterning in the cochlea.
Katsunuma S, Togashi H, Kuno S, Fujita T, Nibu KI. Katsunuma S, et al. Front Cell Dev Biol. 2022 Dec 8;10:1073830. doi: 10.3389/fcell.2022.1073830. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36568980 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01-DC009255/DC/NIDCD NIH HHS/United States
- R01-DC008853/DC/NIDCD NIH HHS/United States
- R01 DC008853/DC/NIDCD NIH HHS/United States
- R01 DC005439/DC/NIDCD NIH HHS/United States
- Z01-DC000060-10/DC/NIDCD NIH HHS/United States
- R01-DC002390/DC/NIDCD NIH HHS/United States
- Z01-DC000021-18/DC/NIDCD NIH HHS/United States
- Z01 DC000021/ImNIH/Intramural NIH HHS/United States
- R01 DC009255/DC/NIDCD NIH HHS/United States
- Z01 DC000060/ImNIH/Intramural NIH HHS/United States
- R01-DC05439/DC/NIDCD NIH HHS/United States
- R01 DC002390/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases